Diagnostic accuracy of a point-of-care urine test for tuberculosis screening among newly-diagnosed HIV-infected adults: a prospective, clinic-based study
- PMID: 24571362
- PMCID: PMC3944827
- DOI: 10.1186/1471-2334-14-110
Diagnostic accuracy of a point-of-care urine test for tuberculosis screening among newly-diagnosed HIV-infected adults: a prospective, clinic-based study
Abstract
Background: A rapid diagnostic test for active tuberculosis (TB) at the clinical point-of-care could expedite case detection and accelerate TB treatment initiation. We assessed the diagnostic accuracy of a rapid urine lipoarabinomannan (LAM) test for TB screening among HIV-infected adults in a TB-endemic setting.
Methods: We prospectively enrolled newly-diagnosed HIV-infected adults (≥18 years) at 4 outpatient clinics in Durban from Oct 2011-May 2012, excluding those on TB therapy. A physician evaluated all participants and offered CD4 cell count testing. Trained study nurses collected a sputum sample for acid-fast bacilli smear microscopy (AFB) and mycobacterial culture, and performed urine LAM testing using Determine™ TB LAM in the clinic. The presence of a band regardless of intensity on the urine LAM test was considered positive. We defined as the gold standard for active pulmonary TB a positive sputum culture for Mycobacterium tuberculosis. Diagnostic accuracy of urine LAM was assessed, alone and in combination with smear microscopy, and stratified by CD4 cell count.
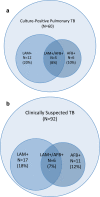
Results: Among 342 newly-diagnosed HIV-infected participants, 190 (56%) were male, mean age was 35.6 years, and median CD4 was 182/mm3. Sixty participants had culture-positive pulmonary TB, resulting in an estimated prevalence of 17.5% (95% CI 13.7-22.0%). Forty-five (13.2%) participants were urine LAM positive. Mean time from urine specimen collection to LAM test result was 40 minutes (95% CI 34-46 minutes). Urine LAM test sensitivity was 28.3% (95% CI 17.5-41.4) overall, and 37.5% (95% CI 21.1-56.3) for those with CD4 count <100/mm3, while specificity was 90.1% (95% CI 86.0-93.3) overall, and 86.9% (95% CI 75.8-94.2) for those with CD4 < 100/mm3. When combined with sputum AFB (either test positive), sensitivity increased to 38.3% (95% CI 26.0-51.8), but specificity decreased to 85.8% (95% CI 81.1-89.7).
Conclusions: In this prospective, clinic-based study with trained nurses, a rapid urine LAM test had low sensitivity for TB screening among newly-diagnosed HIV-infected adults, but improved sensitivity when combined with sputum smear microscopy.
Figures

References
-
- World Health Organization. Global tuberculosis control 2011. Geneva: World Health Organization; 2011.
-
- World Health Organization. Joint TB/HIV Interventions. Geneva: World Health Organization; 2009.
-
- World Health Organization. TB/HIV Working Group. Geneva; World Health: Priority research questions for HIV/TB in HIV-prevalent and resource-limited settings; 2010.
-
- Bassett IV, Wang B, Chetty S, Giddy J, Losina E, Mazibuko M, Bearnot B, Allen J, Walensky RP, Freedberg KA. Intensive tuberculosis screening for HIV-infected patients starting antiretroviral therapy in Durban, South Africa. Clin Infect Dis. 2010;14(7):823–829. doi: 10.1086/656282. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

