Pathogenesis of percutaneous infection of goats with Burkholderia pseudomallei: clinical, pathologic, and immunological responses in chronic melioidosis
- PMID: 24571408
- PMCID: PMC3960038
- DOI: 10.1111/iep.12068
Pathogenesis of percutaneous infection of goats with Burkholderia pseudomallei: clinical, pathologic, and immunological responses in chronic melioidosis
Abstract
Melioidosis is a severe suppurative to granulomatous infection caused by Burkholderia pseudomallei. The disease is endemic to South-East Asia and Northern Australasia and is also of interest as a potential biological weapon. Natural infection can occur by percutaneous inoculation, inhalation or ingestion, but the relative importance of each route is unknown. Experimental infection models using mice have shown inhalation to be the most lethal route of exposure, but few studies have examined the pathogenesis of percutaneous infection despite its presumptive importance in natural disease. Caprine models are useful in the study of melioidosis because goats are susceptible to natural infection by B. pseudomallei, display similar epizootiology/epidemiology to that of humans within the endemic range and develop similar pathologic lesions. Percutaneous inoculation with 10(4) CFU of B. pseudomallei produced disease in all experimental animals with rapid dissemination to the lungs, spleen and kidneys. Initial fever was brief, but temperatures did not return to pre-infection levels until day 18, concurrent with a dramatic lymphocytosis and the transition to chronic disease. Distribution and appearance of gross pathologic and radiographic lesions in goats were similar to caprine aerosol infection and to reported human disease. The similarities seen despite different routes of infection suggest that host or bacterial factors may be more important than the route of infection in disease pathogenesis. The nature of melioidosis in goats makes it amenable for modelling additional risk factors to produce acute clinical disease, which is important to the study of human melioidosis.
Keywords: Burkholderia pseudomallei; goat; melioidosis; model; pathogenesis.
© 2014 The Authors. International Journal of Experimental Pathology © 2014 International Journal of Experimental Pathology.
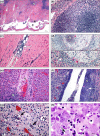
Figures
 ), BpG49 (
), BpG49 ( ), BpG56 (
), BpG56 ( ), BpG57 (□).
), BpG57 (□).
 ) showed an initial peak on day 2 and then on day 18 before a significant decrease on day 32 and a return to pre-infection levels on day 39. Lymphocytes (
) showed an initial peak on day 2 and then on day 18 before a significant decrease on day 32 and a return to pre-infection levels on day 39. Lymphocytes ( ) first increase over pre-infection levels on day 11, with a significant increase out of the normal range on day 18, which is associated with a significant decrease in temperature (
) first increase over pre-infection levels on day 11, with a significant increase out of the normal range on day 18, which is associated with a significant decrease in temperature ( ) to pre-infection levels. Lymphocytes significantly decrease to pre-infection levels on day 32. (‡) Different than previous measure and pre-infection, (†) different than previous measure but no different than pre-infection, (*) different than pre-infection, (ø) not different than previous measure or pre-infection, significance P < 0.05.
) to pre-infection levels. Lymphocytes significantly decrease to pre-infection levels on day 32. (‡) Different than previous measure and pre-infection, (†) different than previous measure but no different than pre-infection, (*) different than pre-infection, (ø) not different than previous measure or pre-infection, significance P < 0.05.
 ) and percutaneous (
) and percutaneous ( ) groups. Significant elevations were only present for CCL2 in aerosol-infected goats and IFNγ in percutaneously infected goats. TGF-β1 shows an initial decrease followed by increases through day 21 before returning to pre-infection levels on day 42. (‡) Different than previous measure and pre-infection, (†) different than previous measure but no different than pre-infection, (*) different than pre-infection, (ø) not different than previous measure or pre-infection, significance P < 0.05.
) groups. Significant elevations were only present for CCL2 in aerosol-infected goats and IFNγ in percutaneously infected goats. TGF-β1 shows an initial decrease followed by increases through day 21 before returning to pre-infection levels on day 42. (‡) Different than previous measure and pre-infection, (†) different than previous measure but no different than pre-infection, (*) different than pre-infection, (ø) not different than previous measure or pre-infection, significance P < 0.05.References
-
- Barnes JL, Warner J, Melrose W, et al. Adaptive immunity in melioidosis: a possible role for T cells in determining outcome of infection with Burkholderia pseudomallei. Clin. Immunol. 2004;113:22–28. - PubMed
-
- Bateson EM, Webling DD. The radiological appearances of pulmonary melioidosis: a report on twenty-three cases. Australas. Radiol. 1981;25:239–245. - PubMed
-
- Cheng AC, Jacups SP, Gal D, Mayo M, Currie BJ. Extreme weather events and environmental contamination are associated with case-clusters of melioidosis in the Northern Territory of Australia. Int. J. Epidemiol. 2006;35:323–329. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

