Antitumor effects of flavopiridol on human uterine leiomyoma in vitro and in a xenograft model
- PMID: 24572052
- PMCID: PMC4212340
- DOI: 10.1177/1933719114525266
Antitumor effects of flavopiridol on human uterine leiomyoma in vitro and in a xenograft model
Abstract
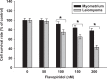
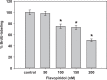
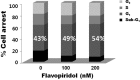
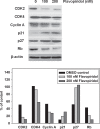
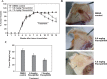
Dysregulated cyclin-dependent kinases (CDKs) are considered a potential target for cancer therapy. Flavopiridol is a potent CDK inhibitor. In this study, the antiproliferative effect of the flavonoid compound flavopiridol and its mechanism in human uterine leiomyoma cells were investigated. The present study focused on the effect of flavopiridol in cell proliferation and cell cycle progression in primary cultured human uterine leiomyoma cells. Cell viability and cell proliferation assays were conducted. Flow cytometry was performed to determine the effect of flavopiridol on cell cycle. The expression of cell cycle regulatory-related proteins was evaluated by Western blotting. Cell viability and proliferation of uterine leiomyoma cells were significantly reduced by flavopiridol treatment in a dose-dependent manner. Flow cytometry results showed that flavopiridol induced G1 phase arrest. Flavopiridol-induced growth inhibition in uterine leiomyoma cells was associated with increased expression of p21(cip/wafl) and p27(kip1) in a dose-dependent manner. Downregulation of CDK2/4 and Cyclin A with a concomitant increase in dephosphorylation of retinoblastoma was observed. This study demonstrates that flavopiridol inhibits cell proliferation by initiating G1 cell cycle arrest in human uterine leiomyoma. We also found that flavopiridol is effective in inhibiting xenografted human uterine leiomyoma growth. These results indicate that flavopiridol could prove to be a promising chemopreventive and therapeutic agent for human uterine leiomyoma.
Keywords: flavopiridol; leiomyoma; p27kip1; uterus; xenograft.
© The Author(s) 2014.
Conflict of interest statement
Figures

Similar articles
-
Induction of growth inhibition and apoptosis in human uterine leiomyoma cells by isoliquiritigenin.Reprod Sci. 2008 Jul;15(6):552-8. doi: 10.1177/1933719107312681. Epub 2008 May 16. Reprod Sci. 2008. PMID: 18487228
-
Halofuginone suppresses growth of human uterine leiomyoma cells in a mouse xenograft model.Hum Reprod. 2016 Jul;31(7):1540-51. doi: 10.1093/humrep/dew094. Epub 2016 Apr 29. Hum Reprod. 2016. PMID: 27130615 Free PMC article.
-
Rhabdoid tumor growth is inhibited by flavopiridol.Clin Cancer Res. 2008 Jan 15;14(2):523-32. doi: 10.1158/1078-0432.CCR-07-1347. Clin Cancer Res. 2008. PMID: 18223228
-
Flavopiridol: the first cyclin-dependent kinase inhibitor in human clinical trials.Invest New Drugs. 1999;17(3):313-20. doi: 10.1023/a:1006353008903. Invest New Drugs. 1999. PMID: 10665481 Review.
-
Review of flavopiridol, a cyclin-dependent kinase inhibitor, as breast cancer therapy.Semin Oncol. 2002 Jun;29(3 Suppl 11):77-85. doi: 10.1053/sonc.2002.34059. Semin Oncol. 2002. PMID: 12138401 Review.
Cited by
-
MiR-150-5p May Contribute to Pathogenesis of Human Leiomyoma via Regulation of the Akt/p27Kip1 Pathway In Vitro.Int J Mol Sci. 2019 May 31;20(11):2684. doi: 10.3390/ijms20112684. Int J Mol Sci. 2019. PMID: 31159158 Free PMC article.
-
Effect of 2-methoxyestradiol on SK-LMS-1 uterine leiomyosarcoma cells.Oncol Lett. 2017 Jul;14(1):103-110. doi: 10.3892/ol.2017.6165. Epub 2017 May 12. Oncol Lett. 2017. PMID: 28693141 Free PMC article.
-
G2/M inhibitors as pharmacotherapeutic opportunities for glioblastoma: the old, the new, and the future.Cancer Biol Med. 2018 Nov;15(4):354-374. doi: 10.20892/j.issn.2095-3941.2018.0030. Cancer Biol Med. 2018. PMID: 30766748 Free PMC article.
-
Recent Advances on Small-Molecule Survivin Inhibitors.Curr Med Chem. 2015 Jan 13;22(9):1136 - 1146. doi: 10.2174/0929867322666150114102146. Curr Med Chem. 2015. PMID: 25613234 Free PMC article.
-
Therapeutic Potential of the Cyclin-Dependent Kinase Inhibitor Flavopiridol on c-Myc Overexpressing Esophageal Cancer.Front Pharmacol. 2021 Sep 21;12:746385. doi: 10.3389/fphar.2021.746385. eCollection 2021. Front Pharmacol. 2021. PMID: 34621175 Free PMC article.
References
-
- Catherino WH, Malik M. Uterine leiomyoma express a molecular pattern that lowers retinoic acid exposure. Fertil Steril. 2007;87(6):1388–1398 - PubMed
-
- Corbin A, Beattie CW. Inhibition of the pre-ovulatory proestrous gonadotropin surge, ovulation and pregnancy with a peptide analogue of luteinizing hormone releasing hormone. Endocr Res Commun. 1975;2(1):1–23 - PubMed
-
- Flierman PA, Oberye JJ, Van der Hulst VP, de Blok S. Rapid reduction of leiomyoma volume during treatment with the GnRH antagonist ganirelix. BJOG. 2005;112(5):638–642 - PubMed
-
- Donnez J, Tomaszewski J, Vazquez F, et al. . Ulipristal acetate versus leuprolide acetate for uterine fibroids. N Engl J Med. 2012;366(5):421–432 - PubMed
-
- Grant S, Dent P. Gene profiling and the cyclin-dependent kinase inhibitor flavopiridol: what's in a name? Mol Cancer Ther. 2004;3(7):873–875 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

