Comparison of the neuropoietic activity of gene-modified versus parental mesenchymal stromal cells and the identification of soluble and extracellular matrix-related neuropoietic mediators
- PMID: 24572070
- PMCID: PMC4055059
- DOI: 10.1186/scrt418
Comparison of the neuropoietic activity of gene-modified versus parental mesenchymal stromal cells and the identification of soluble and extracellular matrix-related neuropoietic mediators
Abstract
Introduction: Transplanting mesenchymal stromal cells (MSCs) or their derivatives into a neurodegenerative environment is believed to be beneficial because of the trophic support, migratory guidance, immunosuppression, and neurogenic stimuli they provide. SB623, a cell therapy for the treatment of chronic stroke, currently in a clinical trial, is derived from bone marrow MSCs by using transient transfection with a vector encoding the human Notch1 intracellular domain. This creates a new phenotype, which is effective in experimental stroke, exhibits immunosuppressive and angiogenic activity equal or superior to parental MSCs in vitro, and produces extracellular matrix (ECM) that is exceptionally supportive for neural cell growth. The neuropoietic activity of SB623 and parental MSCs has not been compared, and the SB623-derived neuropoietic mediators have not been identified.
Methods: SB623 or parental MSCs were cocultured with rat embryonic brain cortex cells on cell-derived ECM in a previously characterized quantitative neuropoiesis assay. Changes in expression of rat neural differentiation markers were quantified by using rat-specific qRT-PCR. Human mediators were identified by using expression profiling, an enzymatic crosslinking activity, and functional interference studies by means of blocking antibodies, biologic inhibitors, and siRNA. Cocultures were immunolabeled for presynaptic vesicular transporters to assess neuronal specialization.
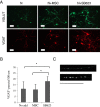
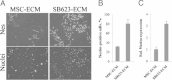
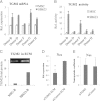
Results: Among six MSC/SB623 pairs, SB623 induced expression of rat neural precursor, oligodendrocyte, and astrocyte markers on average 2.6 to 3 times stronger than did their parental MSCs. SB623 expressed significantly higher FGF2, FGF1, and BMP4, and lower FGFR1 and FGFR2 levels; and human FGF1, FGF2, BMPs, and HGF were implicated as neuropoietic mediators. Neural precursors grew faster on SB623- than on MSC-derived ECM. SB623 exhibited higher expression levels and crosslinking activity of tissue transglutaminase (TGM2). TGM2 silencing reduced neural precursor growth on SB623-ECM. SB623 also promoted the induction of GABA-ergic, but not glutamatergic, neurons more effectively than did MSCs.
Conclusions: These data demonstrate that SB623 cells tend to support neural cell growth more effectively than their parental MSCs and identify both soluble and insoluble mediators responsible, at least in part, for enhanced neuropoietic potency of SB623. The neuropoiesis assay is a useful tool for identifying beneficial factors produced by MSCs and their derivatives.
Figures


Similar articles
-
Quantitative microplate assay for studying mesenchymal stromal cell-induced neuropoiesis.Stem Cells Transl Med. 2013 Mar;2(3):223-32. doi: 10.5966/sctm.2012-0119. Epub 2013 Feb 19. Stem Cells Transl Med. 2013. PMID: 23430693 Free PMC article.
-
Extracellular matrix produced by bone marrow stromal cells and by their derivative, SB623 cells, supports neural cell growth.J Neurosci Res. 2009 Nov 1;87(14):3198-206. doi: 10.1002/jnr.22146. J Neurosci Res. 2009. PMID: 19530164
-
Comparing the angiogenic potency of naïve marrow stromal cells and Notch-transfected marrow stromal cells.J Transl Med. 2013 Mar 27;11:81. doi: 10.1186/1479-5876-11-81. J Transl Med. 2013. PMID: 23531336 Free PMC article.
-
Electrophysiological properties and synaptic function of mesenchymal stem cells during neurogenic differentiation - a mini-review.Int J Artif Organs. 2012 May;35(5):323-37. doi: 10.5301/ijao.5000085. Int J Artif Organs. 2012. PMID: 22505200 Review.
-
Extracellular matrix-induced signaling pathways in mesenchymal stem/stromal cells.Cell Commun Signal. 2023 Sep 19;21(1):244. doi: 10.1186/s12964-023-01252-8. Cell Commun Signal. 2023. PMID: 37726815 Free PMC article. Review.
Cited by
-
Mesenchymal stem cells as a multimodal treatment for nervous system diseases.Stem Cells Transl Med. 2020 Oct;9(10):1174-1189. doi: 10.1002/sctm.19-0430. Epub 2020 Jun 23. Stem Cells Transl Med. 2020. PMID: 32573961 Free PMC article. Review.
-
Transplantation of modified human bone marrow-derived stromal cells affords therapeutic effects on cerebral ischemia in rats.CNS Neurosci Ther. 2022 Dec;28(12):1974-1985. doi: 10.1111/cns.13947. Epub 2022 Aug 24. CNS Neurosci Ther. 2022. PMID: 36000240 Free PMC article.
-
Harnessing the Secretome of Mesenchymal Stromal Cells for Traumatic Spinal Cord Injury: Multicell Comparison and Assessment of In Vivo Efficacy.Stem Cells Dev. 2020 Nov 15;29(22):1429-1443. doi: 10.1089/scd.2020.0079. Epub 2020 Oct 21. Stem Cells Dev. 2020. PMID: 32962528 Free PMC article.
-
Enhancement of matrix metalloproteinases 2 and 9 accompanied with neurogenesis following collagen glycosaminoglycan matrix implantation after surgical brain injury.Neural Regen Res. 2018 Jun;13(6):1007-1012. doi: 10.4103/1673-5374.233443. Neural Regen Res. 2018. PMID: 29926827 Free PMC article.
-
Modified human mesenchymal stromal/stem cells restore cortical excitability after focal ischemic stroke in rats.Mol Ther. 2025 Jan 8;33(1):375-400. doi: 10.1016/j.ymthe.2024.12.006. Epub 2024 Dec 11. Mol Ther. 2025. PMID: 39668560
References
-
- Yamashita T, Ninomiya M, Hernández Acosta P, García-Verdugo JM, Sunabori T, Sakaguchi M, Adachi K, Kojima T, Hirota Y, Kawase T, Araki N, Abe K, Okano H, Sawamoto K. Subventricular zone-derived neuroblasts migrate and differentiate into mature neurons in the post-stroke adult striatum. J Neurosci. 2006;26:6627–6636. doi: 10.1523/JNEUROSCI.0149-06.2006. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

