Non-tuberculous mycobacteria have diverse effects on BCG efficacy against Mycobacterium tuberculosis
- PMID: 24572168
- PMCID: PMC4066954
- DOI: 10.1016/j.tube.2013.12.006
Non-tuberculous mycobacteria have diverse effects on BCG efficacy against Mycobacterium tuberculosis
Abstract
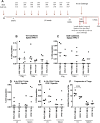
The efficacy of Bacillus Calmette-Guerin (BCG) vaccination in protection against pulmonary tuberculosis (TB) is highly variable between populations. One possible explanation for this variability is increased exposure of certain populations to non-tuberculous mycobacteria (NTM). This study used a murine model to determine the effect that exposure to NTM after BCG vaccination had on the efficacy of BCG against aerosol Mycobacterium tuberculosis challenge. The effects of administering live Mycobacterium avium (MA) by an oral route and killed MA by a systemic route on BCG-induced protection were evaluated. CD4+ and CD8+ T cell responses were profiled to define the immunological mechanisms underlying any effect on BCG efficacy. BCG efficacy was enhanced by exposure to killed MA administered by a systemic route; T helper 1 and T helper 17 responses were associated with increased protection. BCG efficacy was reduced by exposure to live MA administered by the oral route; T helper 2 cells were associated with reduced protection. These findings demonstrate that exposure to NTM can induce opposite effects on BCG efficacy depending on route of exposure and viability of NTM. A reproducible model of NTM exposure would be valuable in the evaluation of novel TB vaccine candidates.
Keywords: Bacillus Calmette-Geurin; Mouse model; Mycobacterium avium; Non-tuberculous mycobacteria; Tuberculosis.
Copyright © 2014 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures









References
-
- Organisation WH . WHO Library Cataloguing in Publication Data; 2012. Global tuberculosis report 2012.
-
- Walzl G., Ronacher K., Hanekom W., Scriba T.J., Zumla A. Immunological biomarkers of tuberculosis. Nat Rev Immunol. 2011;11:343–354. - PubMed
-
- Begum D., Umemura M., Yahagi A., Okamoto Y., Hamada S., Oshiro K., Matsuzaki G. Accelerated induction of mycobacterial antigen-specific CD8+ T cells in the Mycobacterium tuberculosis-infected lung by subcutaneous vaccination with Mycobacterium bovis bacille Calmette-Guerin. Immunology. 2009;128:556–563. - PMC - PubMed
-
- Hope J.C., Kwong L.S., Sopp P., Collins R.A., Howard C.J. Dendritic cells induce CD4+ and CD8+ T-cell responses to Mycobacterium bovis and M. avium antigens in Bacille Calmette Guerin vaccinated and nonvaccinated cattle. Scand J Immunol. 2000;52:285–291. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

