Autoimmune basis for postural tachycardia syndrome
- PMID: 24572257
- PMCID: PMC3959717
- DOI: 10.1161/JAHA.113.000755
Autoimmune basis for postural tachycardia syndrome
Abstract
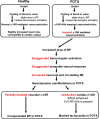
Background: Patients with postural tachycardia syndrome (POTS) have exaggerated orthostatic tachycardia often following a viral illness, suggesting autoimmunity may play a pathophysiological role in POTS. We tested the hypothesis that they harbor functional autoantibodies to adrenergic receptors (AR).
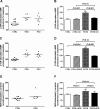
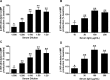
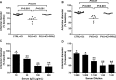
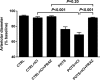
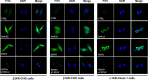
Methods and results: Fourteen POTS patients (7 each from 2 institutions) and 10 healthy subjects were examined for α1AR autoantibody-mediated contractility using a perfused rat cremaster arteriole assay. A receptor-transfected cell-based assay was used to detect the presence of β1AR and β2AR autoantibodies. Data were normalized and expressed as a percentage of baseline. The sera of all 14 POTS patients demonstrated significant arteriolar contractile activity (69±3% compared to 91±1% of baseline for healthy controls, P<0.001) when coexisting β2AR dilative activity was blocked; and this was suppressed by α1AR blockade with prazosin. POTS sera acted as a partial α1AR antagonist significantly shifting phenylephrine contractility curves to the right. All POTS sera increased β1AR activation (130±3% of baseline, P<0.01) and a subset had increased β2AR activity versus healthy subjects. POTS sera shifted isoproterenol cAMP response curves to the left, consistent with enhanced β1AR and β2AR agonist activity. Autoantibody-positive POTS sera demonstrated specific binding to β1AR, β2AR, and α1AR in transfected cells.
Conclusions: POTS patients have elevated α1AR autoantibodies exerting a partial peripheral antagonist effect resulting in a compensatory sympathoneural activation of α1AR for vasoconstriction and concurrent βAR-mediated tachycardia. Coexisting β1AR and β2AR agonistic autoantibodies facilitate this tachycardia. These findings may explain the increased standing plasma norepinephrine and excessive tachycardia observed in many POTS patients.
Keywords: adrenergic receptor; autoantibody; autonomic function; postural tachycardia syndrome.
Figures

References
-
- Schondorf R, Low PA. Idiopathic postural orthostatic tachycardia syndrome: an attenuated form of acute pandysautonomia? Neurology. 1993; 43:132-137 - PubMed
-
- Grubb BP. Postural tachycardia syndrome. Circulation. 2008; 117:2814-2817 - PubMed
-
- Robertson D. The epidemic of orthostatic tachycardia and orthostatic intolerance. Am J Med Sci. 1999; 317:75-77 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

