Aptamer against mannose-capped lipoarabinomannan inhibits virulent Mycobacterium tuberculosis infection in mice and rhesus monkeys
- PMID: 24572295
- PMCID: PMC4015227
- DOI: 10.1038/mt.2014.31
Aptamer against mannose-capped lipoarabinomannan inhibits virulent Mycobacterium tuberculosis infection in mice and rhesus monkeys
Abstract
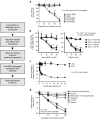
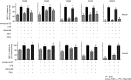
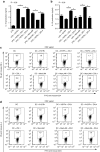
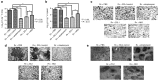
The major surface lipoglycan of Mycobacterium tuberculosis (M. tb), mannose-capped lipoarabinomannan (ManLAM), is an immunosuppressive epitope of M. tb. We used systematic evolution of ligands by exponential enrichment (SELEX) to generate an aptamer (ZXL1) that specifically bound to ManLAM from the virulent M. tb strain H37Rv. Aptamer ZXL1 had the highest binding affinity, with an equilibrium dissociation constant (Kd) of 436.3 ± 37.84 nmol/l, and competed with the mannose receptor for binding to ManLAM and M. tb H37Rv. ZXL1 significantly inhibited the ManLAM-induced immunosuppression of CD11c(+) dendritic cells (DCs) and enhanced the M. tb antigen-presenting activity of DCs for naive CD4(+) Th1 cell activation. More importantly, we demonstrated that injection of aptamer ZXL1 significantly reduced the progression of M. tb H37Rv infections and bacterial loads in lungs of mice and rhesus monkeys. These results suggest that the aptamer ZXL1 is a new potential antimycobacterial agent and tuberculosis vaccine immune adjuvant.
Figures



Similar articles
-
A single-stranded DNA aptamer against mannose-capped lipoarabinomannan enhances anti-tuberculosis activity of macrophages through downregulation of lipid-sensing nuclear receptor peroxisome proliferator-activated receptor γ expression.Microbiol Immunol. 2017 Feb;61(2):92-102. doi: 10.1111/1348-0421.12470. Microbiol Immunol. 2017. PMID: 28206680
-
Generation and application of ssDNA aptamers against glycolipid antigen ManLAM of Mycobacterium tuberculosis for TB diagnosis.J Infect. 2016 May;72(5):573-86. doi: 10.1016/j.jinf.2016.01.014. Epub 2016 Feb 3. J Infect. 2016. PMID: 26850356
-
A Single ssDNA Aptamer Binding to Mannose-Capped Lipoarabinomannan of Bacillus Calmette-Guérin Enhances Immunoprotective Effect against Tuberculosis.J Am Chem Soc. 2016 Sep 14;138(36):11680-9. doi: 10.1021/jacs.6b05357. Epub 2016 Aug 29. J Am Chem Soc. 2016. PMID: 27529508
-
Mannose-capped lipoarabinomannan in Mycobacterium tuberculosis pathogenesis.Pathog Dis. 2018 Jun 1;76(4):fty026. doi: 10.1093/femspd/fty026. Pathog Dis. 2018. PMID: 29722821 Free PMC article. Review.
-
Mycobacterial mannose-capped lipoarabinomannan: a modulator bridging innate and adaptive immunity.Emerg Microbes Infect. 2019;8(1):1168-1177. doi: 10.1080/22221751.2019.1649097. Emerg Microbes Infect. 2019. PMID: 31379262 Free PMC article. Review.
Cited by
-
Manipulation of the endocytic pathway and phagocyte functions by Mycobacterium tuberculosis lipoarabinomannan.Front Cell Infect Microbiol. 2015 Jan 12;4:187. doi: 10.3389/fcimb.2014.00187. eCollection 2014. Front Cell Infect Microbiol. 2015. PMID: 25629008 Free PMC article. Review.
-
Mannose-capped Lipoarabinomannan from Mycobacterium tuberculosis induces IL-37 production via upregulating ERK1/2 and p38 in human type II alveolar epithelial cells.Int J Clin Exp Med. 2015 May 15;8(5):7279-87. eCollection 2015. Int J Clin Exp Med. 2015. PMID: 26221267 Free PMC article.
-
Oligonucleotide aptamers: promising and powerful diagnostic and therapeutic tools for infectious diseases.J Infect. 2018 Aug;77(2):83-98. doi: 10.1016/j.jinf.2018.04.007. Epub 2018 May 7. J Infect. 2018. PMID: 29746951 Free PMC article. Review.
-
Aptamers: An Emerging Tool for Diagnosis and Therapeutics in Tuberculosis.Front Cell Infect Microbiol. 2021 Jul 1;11:656421. doi: 10.3389/fcimb.2021.656421. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34277465 Free PMC article. Review.
-
Essential Roles of PPARs in Lipid Metabolism during Mycobacterial Infection.Int J Mol Sci. 2021 Jul 15;22(14):7597. doi: 10.3390/ijms22147597. Int J Mol Sci. 2021. PMID: 34299217 Free PMC article. Review.
References
-
- World Health Organization. 2011The burden of disease caused by TB Global tuberculosis control 2011. ( http://whqlibdoc.who.int/publications/2011/9789241564380_eng.pdf ).
-
- Svenson S, Källenius G, Pawlowski A, Hamasur B. Towards new tuberculosis vaccines. Hum Vaccin. 2010;6:309–317. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

