Mouse liver repopulation with hepatocytes generated from human fibroblasts
- PMID: 24572354
- PMCID: PMC4161230
- DOI: 10.1038/nature13020
Mouse liver repopulation with hepatocytes generated from human fibroblasts
Abstract
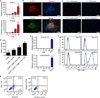
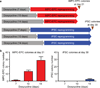
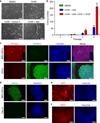
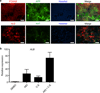
Human induced pluripotent stem cells (iPSCs) have the capability of revolutionizing research and therapy of liver diseases by providing a source of hepatocytes for autologous cell therapy and disease modelling. However, despite progress in advancing the differentiation of iPSCs into hepatocytes (iPSC-Heps) in vitro, cells that replicate the ability of human primary adult hepatocytes (aHeps) to proliferate extensively in vivo have not been reported. This deficiency has hampered efforts to recreate human liver diseases in mice, and has cast doubt on the potential of iPSC-Heps for liver cell therapy. The reason is that extensive post-transplant expansion is needed to establish and sustain a therapeutically effective liver cell mass in patients, a lesson learned from clinical trials of aHep transplantation. Here, as a solution to this problem, we report the generation of human fibroblast-derived hepatocytes that can repopulate mouse livers. Unlike current protocols for deriving hepatocytes from human fibroblasts, ours did not generate iPSCs but cut short reprogramming to pluripotency to generate an induced multipotent progenitor cell (iMPC) state from which endoderm progenitor cells and subsequently hepatocytes (iMPC-Heps) could be efficiently differentiated. For this purpose we identified small molecules that aided endoderm and hepatocyte differentiation without compromising proliferation. After transplantation into an immune-deficient mouse model of human liver failure, iMPC-Heps proliferated extensively and acquired levels of hepatocyte function similar to those of aHeps. Unfractionated iMPC-Heps did not form tumours, most probably because they never entered a pluripotent state. Our results establish the feasibility of significant liver repopulation of mice with human hepatocytes generated in vitro, which removes a long-standing roadblock on the path to autologous liver cell therapy.
Conflict of interest statement
The authors declare no competing financial interests.
Figures

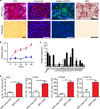


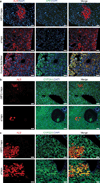


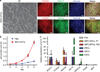
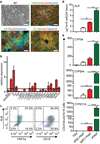

Comment in
-
Regenerative medicine. Knocking on the door to successful hepatocyte transplantation.Nat Rev Gastroenterol Hepatol. 2014 May;11(5):277-8. doi: 10.1038/nrgastro.2014.44. Epub 2014 Mar 25. Nat Rev Gastroenterol Hepatol. 2014. PMID: 24662276
References
-
- Puppi J, et al. Improving the techniques for human hepatocyte transplantation: report from a consensus meeting in London. Cell Transplant. 2012;21:1–10. - PubMed
Publication types
MeSH terms
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

