Crystal structure of the plant dual-affinity nitrate transporter NRT1.1
- PMID: 24572362
- PMCID: PMC3968801
- DOI: 10.1038/nature13074
Crystal structure of the plant dual-affinity nitrate transporter NRT1.1
Abstract
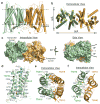
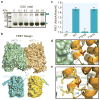
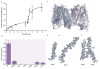
Nitrate is a primary nutrient for plant growth, but its levels in soil can fluctuate by several orders of magnitude. Previous studies have identified Arabidopsis NRT1.1 as a dual-affinity nitrate transporter that can take up nitrate over a wide range of concentrations. The mode of action of NRT1.1 is controlled by phosphorylation of a key residue, Thr 101; however, how this post-translational modification switches the transporter between two affinity states remains unclear. Here we report the crystal structure of unphosphorylated NRT1.1, which reveals an unexpected homodimer in the inward-facing conformation. In this low-affinity state, the Thr 101 phosphorylation site is embedded in a pocket immediately adjacent to the dimer interface, linking the phosphorylation status of the transporter to its oligomeric state. Using a cell-based fluorescence resonance energy transfer assay, we show that functional NRT1.1 dimerizes in the cell membrane and that the phosphomimetic mutation of Thr 101 converts the protein into a monophasic high-affinity transporter by structurally decoupling the dimer. Together with analyses of the substrate transport tunnel, our results establish a phosphorylation-controlled dimerization switch that allows NRT1.1 to uptake nitrate with two distinct affinity modes.
Conflict of interest statement
Authors declare no financial interest.
Figures


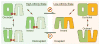






Comment in
-
Plant science: How to switch affinity.Nature. 2014 Mar 6;507(7490):44-5. doi: 10.1038/nature13063. Epub 2014 Feb 26. Nature. 2014. PMID: 24572361 No abstract available.
References
-
- Nacry PB, Gojon EA. Nitrogen acquisition by roots: physiological and developmental mechanisms ensuring plant adaptation to a fluctuating resource. Plant and Soil. 2013;370:29. doi: 10.1007/s11104-013-1645-9. - DOI
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

