IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases
- PMID: 24572363
- PMCID: PMC4260166
- DOI: 10.1038/nature12979
IL-35-producing B cells are critical regulators of immunity during autoimmune and infectious diseases
Abstract
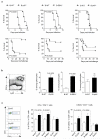
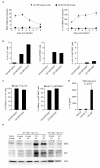
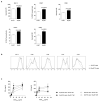
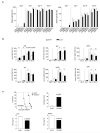
B lymphocytes have critical roles as positive and negative regulators of immunity. Their inhibitory function has been associated primarily with interleukin 10 (IL-10) because B-cell-derived IL-10 can protect against autoimmune disease and increase susceptibility to pathogens. Here we identify IL-35-producing B cells as key players in the negative regulation of immunity. Mice in which only B cells did not express IL-35 lost their ability to recover from the T-cell-mediated demyelinating autoimmune disease experimental autoimmune encephalomyelitis (EAE). In contrast, these mice displayed a markedly improved resistance to infection with the intracellular bacterial pathogen Salmonella enterica serovar Typhimurium as shown by their superior containment of the bacterial growth and their prolonged survival after primary infection, and upon secondary challenge, compared to control mice. The increased immunity found in mice lacking IL-35 production by B cells was associated with a higher activation of macrophages and inflammatory T cells, as well as an increased function of B cells as antigen-presenting cells (APCs). During Salmonella infection, IL-35- and IL-10-producing B cells corresponded to two largely distinct sets of surface-IgM(+)CD138(hi)TACI(+)CXCR4(+)CD1d(int)Tim1(int) plasma cells expressing the transcription factor Blimp1 (also known as Prdm1). During EAE, CD138(+) plasma cells were also the main source of B-cell-derived IL-35 and IL-10. Collectively, our data show the importance of IL-35-producing B cells in regulation of immunity and highlight IL-35 production by B cells as a potential therapeutic target for autoimmune and infectious diseases. This study reveals the central role of activated B cells, particularly plasma cells, and their production of cytokines in the regulation of immune responses in health and disease.
Figures









References
-
- Fillatreau S, Gray D, Anderton SM. Not always the bad guys: B cells as regulators of autoimmune pathology. Nature reviews. Immunology. 2008;8:391–397. doi:10.1038/nri2315. - PubMed
-
- Fillatreau S. Novel regulatory functions for Toll-like receptor-activated B cells during intracellular bacterial infection. Immunological reviews. 2011;240:52–71. doi:10.1111/j.1600-065X.2010.00991.x. - PubMed
-
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nature immunology. 2002;3:944–950. doi:10.1038/ni833. - PubMed
-
- Mizoguchi A, Mizoguchi E, Takedatsu H, Blumberg RS, Bhan AK. Chronic intestinal inflammatory condition generates IL-10-producing regulatory B cell subset characterized by CD1d upregulation. Immunity. 2002;16:219–230. - PubMed
Additional references for Methods
-
- Biesen R, et al. Sialic acid-binding Ig-like lectin 1 expression in inflammatory and resident monocytes is a potential biomarker for monitoring disease activity and success of therapy in systemic lupus erythematosus. Arthritis and rheumatism. 2008;58:1136–1145. doi:10.1002/art.23404. - PubMed
-
- Saeed AI, et al. TM4: a free, open-source system for microarray data management and analysis. BioTechniques. 2003;34:374–378. - PubMed
-
- Hoehlig K, et al. Activation of CD4(+) Foxp3(+) regulatory T cells proceeds normally in the absence of B cells during EAE. European journal of immunology. 2012;42:1164–1173. doi:10.1002/eji.201142242. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

