p16INK4a and its regulator miR-24 link senescence and chondrocyte terminal differentiation-associated matrix remodeling in osteoarthritis
- PMID: 24572376
- PMCID: PMC4060445
- DOI: 10.1186/ar4494
p16INK4a and its regulator miR-24 link senescence and chondrocyte terminal differentiation-associated matrix remodeling in osteoarthritis
Abstract
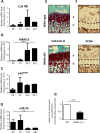
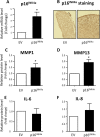
Introduction: Recent evidence suggests that tissue accumulation of senescent p16INK4a-positive cells during the life span would be deleterious for tissue functions and could be the consequence of inherent age-associated disorders. Osteoarthritis (OA) is characterized by the accumulation of chondrocytes expressing p16INK4a and markers of the senescence-associated secretory phenotype (SASP), including the matrix remodeling metalloproteases MMP1/MMP13 and pro-inflammatory cytokines interleukin-8 (IL-8) and IL-6. Here, we evaluated the role of p16INK4a in the OA-induced SASP and its regulation by microRNAs (miRs).
Methods: We used IL-1-beta-treated primary OA chondrocytes cultured in three-dimensional setting or mesenchymal stem cells differentiated into chondrocyte to follow p16INK4a expression. By transient transfection experiments and the use of knockout mice, we validate p16INK4a function in chondrocytes and its regulation by one miR identified by means of a genome-wide miR-array analysis.
Results: p16INK4a is induced upon IL-1-beta treatment and also during in vitro chondrogenesis. In the mouse model, Ink4a locus favors in vivo the proportion of terminally differentiated chondrocytes. When overexpressed in chondrocytes, p16INK4a is sufficient to induce the production of the two matrix remodeling enzymes, MMP1 and MMP13, thus linking senescence with OA pathogenesis and bone development. We identified miR-24 as a negative regulator of p16INK4a. Accordingly, p16INK4a expression increased while miR-24 level was repressed upon IL-1-beta addition, in OA cartilage and during in vitro terminal chondrogenesis.
Conclusions: We disclosed herein a new role of the senescence marker p16INK4a and its regulation by miR-24 during OA and terminal chondrogenesis.
Figures



References
-
- Bachoo RM, Maher EA, Ligon KL, Sharpless NE, Chan SS, You MJ, Tang Y, DeFrances J, Stover E, Weissleder R, Rowitch DH, Louis DN, DePinho RA. Epidermal growth factor receptor and Ink4a/Arf: convergent mechanisms governing terminal differentiation and transformation along the neural stem cell to astrocyte axis. Cancer Cell. 2002;1:269–277. doi: 10.1016/S1535-6108(02)00046-6. - DOI - PubMed
-
- Paramio JM, Segrelles C, Ruiz S, Martin-Caballero J, Page A, Martinez J, Serrano M, Jorcano JL. The ink4a/arf tumor suppressors cooperate with p21cip1/waf in the processes of mouse epidermal differentiation, senescence, and carcinogenesis. J Biol Chem. 2001;276:44203–44211. doi: 10.1074/jbc.M105650200. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

