Analysis of biomolecular interactions using affinity microcolumns: a review
- PMID: 24572459
- PMCID: PMC4112177
- DOI: 10.1016/j.jchromb.2014.01.026
Analysis of biomolecular interactions using affinity microcolumns: a review
Abstract
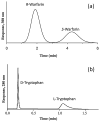
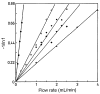
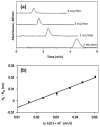
Affinity chromatography has become an important tool for characterizing biomolecular interactions. The use of affinity microcolumns, which contain immobilized binding agents and have volumes in the mid-to-low microliter range, has received particular attention in recent years. Potential advantages of affinity microcolumns include the many analysis and detection formats that can be used with these columns, as well as the need for only small amounts of supports and immobilized binding agents. This review examines how affinity microcolumns have been used to examine biomolecular interactions. Both capillary-based microcolumns and short microcolumns are considered. The use of affinity microcolumns with zonal elution and frontal analysis methods are discussed. The techniques of peak decay analysis, ultrafast affinity extraction, split-peak analysis, and band-broadening studies are also explored. The principles of these methods are examined and various applications are provided to illustrate the use of these methods with affinity microcolumns. It is shown how these techniques can be utilized to provide information on the binding strength and kinetics of an interaction, as well as on the number and types of binding sites. It is further demonstrated how information on competition or displacement effects can be obtained by these methods.
Keywords: Affinity microcolumns; Biointeraction analysis; Frontal affinity chromatography; High-performance affinity chromatography; Ultrafast affinity extraction; Zonal elution.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures








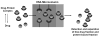
References
-
- Schiel JE, Mallik R, Soman S, Joseph KS, Hage DS. J Sep Sci. 2006;29:719–737. - PubMed
-
- Chaiken IM. Analytical Affinity Chromatography. CRC Press; Boca Raton: 1987.
-
- Hage DS. In: Handbook of HPLC. Corradini D, Katz E, Eksteen R, Shoenmakers P, Miller N, editors. Vol. 4. Marcel Dekker; New York: 1998. pp. 483–498.
-
- Mallik R, Hage DS. J Sep Sci. 2006;29:1686–1704. - PubMed
-
- Kwong TC. Clin Chim Acta. 1985;151:193–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

