ATM specifically mediates repair of double-strand breaks with blocked DNA ends
- PMID: 24572510
- PMCID: PMC3948078
- DOI: 10.1038/ncomms4347
ATM specifically mediates repair of double-strand breaks with blocked DNA ends
Abstract
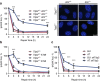
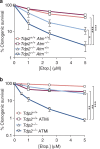
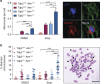
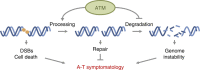
Ataxia telangiectasia is caused by mutations in ATM and represents a paradigm for cancer predisposition and neurodegenerative syndromes linked to deficiencies in the DNA-damage response. The role of ATM as a key regulator of signalling following DNA double-strand breaks (DSBs) has been dissected in extraordinary detail, but the impact of this process on DSB repair still remains controversial. Here we develop novel genetic and molecular tools to modify the structure of DSB ends and demonstrate that ATM is indeed required for efficient and accurate DSB repair, preventing cell death and genome instability, but exclusively when the ends are irreversibly blocked. We therefore identify the nature of ATM involvement in DSB repair, presenting blocked DNA ends as a possible pathogenic trigger of ataxia telangiectasia and related disorders.
Figures



References
-
- McKinnon P. J. & Caldecott K. W. DNA strand break repair and human genetic disease. Ann. Rev. Genomics. Hum. Genet. 8, 37–55 (2007). - PubMed
-
- Lavin M. F. Ataxia-telangiectasia: from a rare disorder to a paradigm for cell signalling and cancer. Nat. Rev. Mol. Cell. Biol. 9, 759–769 (2008). - PubMed
-
- McKinnon P. J. ATM and the molecular pathogenesis of ataxia telangiectasia. Ann. Rev. Pathol. 7, 303–321 (2012). - PubMed
-
- Shiloh Y. & Ziv Y. The ATM protein kinase: regulating the cellular response to genotoxic stress, and more. Nat. Rev. Mol. Cell. Biol. 14, 197–210 (2013). - PubMed
-
- Reynolds J. J. & Stewart G. S. A nervous predisposition to unrepaired DNA double strand breaks. DNA Repair (Amst) 12, 588–599 (2013). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

