Eukaryotic expression, purification and structure/function analysis of native, recombinant CRISP3 from human and mouse
- PMID: 24573035
- PMCID: PMC3936225
- DOI: 10.1038/srep04217
Eukaryotic expression, purification and structure/function analysis of native, recombinant CRISP3 from human and mouse
Abstract
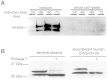
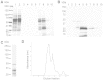
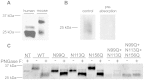
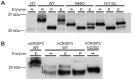
While the Cysteine-Rich Secretory Proteins (CRISPs) have been broadly proposed as regulators of reproduction and immunity, physiological roles have yet to be established for individual members of this family. Past efforts to investigate their functions have been limited by the difficulty of purifying correctly folded CRISPs from bacterial expression systems, which yield low quantities of correctly folded protein containing the eight disulfide bonds that define the CRISP family. Here we report the expression and purification of native, glycosylated CRISP3 from human and mouse, expressed in HEK 293 cells and isolated using ion exchange and size exclusion chromatography. Functional authenticity was verified by substrate-affinity, native glycosylation characteristics and quaternary structure (monomer in solution). Validated protein was used in comparative structure/function studies to characterise sites and patterns of N-glycosylation in CRISP3, revealing interesting inter-species differences.
Figures



Similar articles
-
Purification and characterization of CRISP-3 from human seminal plasma and its real-time binding kinetics with PSP94.J Chromatogr B Analyt Technol Biomed Life Sci. 2016 Dec 15;1039:59-65. doi: 10.1016/j.jchromb.2016.10.032. Epub 2016 Oct 26. J Chromatogr B Analyt Technol Biomed Life Sci. 2016. PMID: 27825912
-
Positive Selection in the Evolution of Mammalian CRISPs.J Mol Evol. 2018 Dec;86(9):635-645. doi: 10.1007/s00239-018-9872-6. Epub 2018 Oct 28. J Mol Evol. 2018. PMID: 30370448
-
Endometrial CRISP3 is regulated throughout the mouse estrous and human menstrual cycle and facilitates adhesion and proliferation of endometrial epithelial cells.Biol Reprod. 2015 Apr;92(4):99. doi: 10.1095/biolreprod.114.127480. Epub 2015 Feb 25. Biol Reprod. 2015. PMID: 25715794
-
Relevance of CRISP proteins for epididymal physiology, fertilization, and fertility.Andrology. 2019 Sep;7(5):610-617. doi: 10.1111/andr.12638. Epub 2019 Jun 19. Andrology. 2019. PMID: 31218833 Review.
-
The functions of CAP superfamily proteins in mammalian fertility and disease.Hum Reprod Update. 2020 Sep 1;26(5):689-723. doi: 10.1093/humupd/dmaa016. Hum Reprod Update. 2020. PMID: 32378701 Review.
Cited by
-
CAP superfamily proteins in human: a new target for cancer therapy.Med Oncol. 2024 Nov 5;41(12):306. doi: 10.1007/s12032-024-02548-6. Med Oncol. 2024. PMID: 39499355 Review.
-
The less conserved metal-binding site in human CRISP1 remains sensitive to zinc ions to permit protein oligomerization.Sci Rep. 2021 Mar 9;11(1):5498. doi: 10.1038/s41598-021-84926-y. Sci Rep. 2021. PMID: 33750840 Free PMC article.
-
CRISPs Function to Boost Sperm Power Output and Motility.Front Cell Dev Biol. 2021 Aug 5;9:693258. doi: 10.3389/fcell.2021.693258. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 34422816 Free PMC article.
-
Secreted venom allergen-like proteins of helminths: Conserved modulators of host responses in animals and plants.PLoS Pathog. 2018 Oct 18;14(10):e1007300. doi: 10.1371/journal.ppat.1007300. eCollection 2018 Oct. PLoS Pathog. 2018. PMID: 30335852 Free PMC article. Review.
References
-
- Gibbs G. M., Roelants K. & O'Bryan M. K. The CAP superfamily: cysteine-rich secretory proteins, antigen 5, and pathogenesis-related 1 proteins--roles in reproduction, cancer, and immune defense. Endocr. Rev. 29, 865–897, 10.1210/er.2008-0032 (2008). - PubMed
-
- Yamazaki Y., Brown R. L. & Morita T. Purification and cloning of toxins from elapid venoms that target cyclic nucleotide-gated ion channels. Biochemistry 41, 11331–11337 (2002). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

