Activation of Notch signaling by short-term treatment with Jagged-1 enhances store-operated Ca(2+) entry in human pulmonary arterial smooth muscle cells
- PMID: 24573085
- PMCID: PMC4010807
- DOI: 10.1152/ajpcell.00221.2013
Activation of Notch signaling by short-term treatment with Jagged-1 enhances store-operated Ca(2+) entry in human pulmonary arterial smooth muscle cells
Abstract
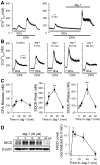
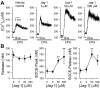
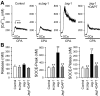
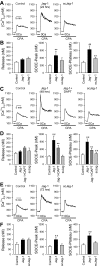
Notch signaling plays a critical role in controlling proliferation and differentiation of pulmonary arterial smooth muscle cells (PASMC). Upregulated Notch ligands and Notch3 receptors in PASMC have been reported to promote the development of pulmonary vascular remodeling in patients with pulmonary arterial hypertension (PAH) and in animals with experimental pulmonary hypertension. Activation of Notch receptors by their ligands leads to the cleavage of the Notch intracellular domain (NICD) to the cytosol by γ-secretase; NICD then translocates into the nucleus to regulate gene transcription. In this study, we examined whether short-term activation of Notch functionally regulates store-operated Ca(2+) entry (SOCE) in human PASMC. Treatment of PASMC with the active fragment of human Jagged-1 protein (Jag-1) for 15-60 min significantly increased the amplitude of SOCE induced by passive deletion of Ca(2+) from the intracellular stores, the sarcoplasmic reticulum (SR). The Jag-1-induced enhancement of SOCE was time dependent: the amplitude was maximized at 30 min of treatment with Jag-1, which was closely correlated with the time course of Jag-1-mediated increase in NICD protein level. The scrambled peptide of Jag-1 active fragment had no effect on SOCE. Inhibition of γ-secretase by N-[N-(3,5-difluorophenacetyl-L-alanyl)]-S-phenylglycine t-butyl ester (DAPT) significantly attenuated the Jag-1-induced augmentation of SOCE. In addition to the short-term effect, prolonged treatment of PASMC with Jag-1 for 48 h also markedly enhanced the amplitude of SOCE. These data demonstrate that short-term activation of Notch signaling enhances SOCE in PASMC; the NICD-mediated functional interaction with store-operated Ca(2+) channels (SOC) may be involved in the Jag-1-mediated enhancement of SOCE in human PASMC.
Keywords: Jagged; Notch intracellular domain; Notch receptor; pulmonary artery; smooth muscle; store-operated calcium entry.
Figures
References
-
- Campos AH, Wang W, Pollman MJ, Gibbons GH. Determinants of Notch-3 receptor expression and signaling in vascular smooth muscle cells: implications in cell-cycle regulation. Circ Res 91: 999–1006, 2002 - PubMed
-
- Chigurupati S, Venkataraman R, Barrera D, Naganathan A, Madan M, Paul L, Pattisapu JV, Kyriazis GA, Sugaya K, Bushnev S, Lathia JD, Rich JN, Chan SL. Receptor channel TRPC6 is a key mediator of Notch-driven glioblastoma growth and invasiveness. Cancer Res 70: 418–427, 2010 - PubMed
-
- Crosnier C, Attie-Bitach T, Encha-Razavi F, Audollent S, Soudy F, Hadchouel M, Meunier-Rotival M, Vekemans M. JAGGED1 gene expression during human embryogenesis elucidates the wide phenotypic spectrum of Alagille syndrome. Hepatology 32: 574–581, 2000 - PubMed
-
- Doi H, Iso T, Shiba Y, Sato H, Yamazaki M, Oyama Y, Akiyama H, Tanaka T, Tomita T, Arai M, Takahashi M, Ikeda U, Kurabayashi M. Notch signaling regulates the differentiation of bone marrow-derived cells into smooth muscle-like cells during arterial lesion formation. Biochem Biophys Res Commun 381: 654–659, 2009 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

