Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging
- PMID: 24573178
- PMCID: PMC4020482
- DOI: 10.3892/ijmm.2014.1663
Biodistribution of mesenchymal stem cell-derived extracellular vesicles in a model of acute kidney injury monitored by optical imaging
Abstract
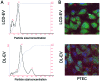
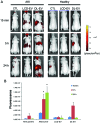
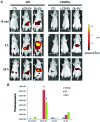
Mesenchymal stem cells (MSCs) contribute to the recovery of tissue injury, providing a paracrine support. Cell-derived extracellular vesicles (EVs), carrying membrane and cytoplasmatic constituents of the cell of origin, have been described as a fundamental mechanism of intercellular communication. We previously demonstrated that EVs derived from human MSCs accelerated recovery following acute kidney injury (AKI) in vivo. The aim of the present study was to investigate the biodistribution and the renal localization of EVs in AKI. For this purpose, two methods for EV labeling suitable for in vivo tracking with optical imaging (OI), were employed using near infrared (NIR) dye (DiD): i) labeled EVs were generated by MSCs pre-incubated with NIR dye and collected from cell supernatants; ii) purified EVs were directly labeled with NIR dye. EVs obtained with these two procedures were injected intravenously (i.v.) into mice with glycerol-induced AKI and into healthy mice to compare the efficacy of the two labeling methods for in vivo detection of EVs at the site of damage. We found that the labeled EVs accumulated specifically in the kidneys of the mice with AKI compared with the healthy controls. After 5 h, the EVs were detectable in whole body images and in dissected kidneys by OI with both types of labeling procedures. The directly labeled EVs showed a higher and brighter fluorescence compared with the labeled EVs produced by cells. The signal generated by the directly labeled EVs was maintained in time, but provided a higher background than that of the labeled EVs produced by cells. The comparison of the two methods indicated that the latter displayed a greater specificity for the injured kidney.
Figures




Similar articles
-
The effects of glomerular and tubular renal progenitors and derived extracellular vesicles on recovery from acute kidney injury.Stem Cell Res Ther. 2017 Feb 7;8(1):24. doi: 10.1186/s13287-017-0478-5. Stem Cell Res Ther. 2017. PMID: 28173878 Free PMC article.
-
Pan PPAR agonist stimulation of induced MSCs produces extracellular vesicles with enhanced renoprotective effect for acute kidney injury.Stem Cell Res Ther. 2024 Jan 2;15(1):9. doi: 10.1186/s13287-023-03577-0. Stem Cell Res Ther. 2024. PMID: 38167146 Free PMC article.
-
Adipose-Derived Mesenchymal Stromal Cells Under Hypoxia: Changes in Extracellular Vesicles Secretion and Improvement of Renal Recovery after Ischemic Injury.Cell Physiol Biochem. 2019;52(6):1463-1483. doi: 10.33594/000000102. Cell Physiol Biochem. 2019. PMID: 31099507
-
Mesenchymal Stem Cell-Derived Extracellular Vesicles to the Rescue of Renal Injury.Int J Mol Sci. 2021 Jun 20;22(12):6596. doi: 10.3390/ijms22126596. Int J Mol Sci. 2021. PMID: 34202940 Free PMC article. Review.
-
Mesenchymal stem cell-derived extracellular vesicles for kidney repair: current status and looming challenges.Stem Cell Res Ther. 2017 Dec 4;8(1):273. doi: 10.1186/s13287-017-0727-7. Stem Cell Res Ther. 2017. PMID: 29202871 Free PMC article. Review.
Cited by
-
Mesenchymal stem/stromal cell-based therapy: mechanism, systemic safety and biodistribution for precision clinical applications.J Biomed Sci. 2021 Apr 14;28(1):28. doi: 10.1186/s12929-021-00725-7. J Biomed Sci. 2021. PMID: 33849537 Free PMC article. Review.
-
The frontier of live tissue imaging across space and time.Cell Stem Cell. 2021 Apr 1;28(4):603-622. doi: 10.1016/j.stem.2021.02.010. Cell Stem Cell. 2021. PMID: 33798422 Free PMC article. Review.
-
Delivery of a model lipophilic membrane cargo to bone marrow via cell-derived microparticles.J Control Release. 2020 Oct 10;326:324-334. doi: 10.1016/j.jconrel.2020.07.019. Epub 2020 Jul 16. J Control Release. 2020. PMID: 32682903 Free PMC article.
-
Molecular Mechanisms of Kidney Injury and Repair.Int J Mol Sci. 2022 Jan 28;23(3):1542. doi: 10.3390/ijms23031542. Int J Mol Sci. 2022. PMID: 35163470 Free PMC article. Review.
-
Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases.Cells. 2019 Dec 11;8(12):1605. doi: 10.3390/cells8121605. Cells. 2019. PMID: 31835680 Free PMC article. Review.
References
-
- Ludwig AK, Giebel B. Exosomes: small vesicles participating in intercellular communication. Int J Biochem Cell Biol. 2012;44:11–15. - PubMed
-
- van Dommelen SM, Vader P, Lakhal S, Kooijmans SA, van Solinge WW, Wood MJ, Schiffelers RM. Microvesicles and exosomes: opportunities for cell-derived membrane vesicles in drug delivery. J Control Release. 2012;161:635–644. - PubMed
-
- Deregibus MC, Cantaluppi V, Calogero R, Lo Iacono M, Tetta C, Biancone L, Bruno S, et al. Endothelial progenitor cell-derived microvescicles activate an angiogenic program in endothelial cells by a horizontal transfer of mRNA. Blood. 2007;110:2440–2448. - PubMed
-
- Valadi H, Ekström K, Bossios A, Sjöstrand M, Lee JJ, Lötvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

