Remembering to eat: hippocampal regulation of meal onset
- PMID: 24573183
- PMCID: PMC4025066
- DOI: 10.1152/ajpregu.00496.2013
Remembering to eat: hippocampal regulation of meal onset
Abstract
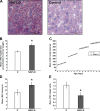
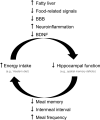
A wide variety of species, including vertebrate and invertebrates, consume food in bouts (i.e., meals). Decades of research suggest that different mechanisms regulate meal initiation (when to start eating) versus meal termination (how much to eat in a meal, also known as satiety). There is a very limited understanding of the mechanisms that regulate meal onset and the duration of the postprandial intermeal interval (ppIMI). In the present review, we examine issues involved in measuring meal onset and some of the limited available evidence regarding how it is regulated. Then, we describe our recent work indicating that dorsal hippocampal neurons inhibit meal onset during the ppIMI and describe the processes that may be involved in this. We also synthesize recent evidence, including evidence from our laboratory, suggesting that overeating impairs hippocampal functioning and that impaired hippocampal functioning, in turn, contributes to the development and/or maintenance of diet-induced obesity. Finally, we identify critical questions and challenges for future research investigating neural controls of meal onset.
Keywords: energy intake; hippocampus; meal onset; memory; obesity.
Copyright © 2014 the American Physiological Society.
Figures
Similar articles
-
Hippocampal neurons inhibit meal onset.Hippocampus. 2013 Jan;23(1):100-7. doi: 10.1002/hipo.22062. Epub 2012 Aug 28. Hippocampus. 2013. PMID: 22927320
-
Cognitive control of meal onset and meal size: Role of dorsal hippocampal-dependent episodic memory.Physiol Behav. 2016 Aug 1;162:112-9. doi: 10.1016/j.physbeh.2016.03.036. Epub 2016 Apr 13. Physiol Behav. 2016. PMID: 27083124 Review.
-
Postmeal Optogenetic Inhibition of Dorsal or Ventral Hippocampal Pyramidal Neurons Increases Future Intake.eNeuro. 2019 Jan 28;6(1):ENEURO.0457-18.2018. doi: 10.1523/ENEURO.0457-18.2018. eCollection 2019 Jan-Feb. eNeuro. 2019. PMID: 30693314 Free PMC article.
-
Memory inhibition and energy regulation.Physiol Behav. 2005 Dec 15;86(5):731-46. doi: 10.1016/j.physbeh.2005.09.004. Epub 2005 Nov 2. Physiol Behav. 2005. PMID: 16263144 Review.
-
Ventral hippocampal neurons inhibit postprandial energy intake.Hippocampus. 2017 Mar;27(3):274-284. doi: 10.1002/hipo.22692. Epub 2017 Jan 25. Hippocampus. 2017. PMID: 28121049
Cited by
-
GLP-1 and weight loss: unraveling the diverse neural circuitry.Am J Physiol Regul Integr Comp Physiol. 2016 May 15;310(10):R885-95. doi: 10.1152/ajpregu.00520.2015. Epub 2016 Mar 30. Am J Physiol Regul Integr Comp Physiol. 2016. PMID: 27030669 Free PMC article. Review.
-
Do impaired memory and body weight regulation originate in childhood with diet-induced hippocampal dysfunction?Am J Clin Nutr. 2014 May;99(5):971-2. doi: 10.3945/ajcn.114.086462. Epub 2014 Mar 26. Am J Clin Nutr. 2014. PMID: 24670945 Free PMC article. No abstract available.
-
Regulation of Memory Function by Feeding-Relevant Biological Systems: Following the Breadcrumbs to the Hippocampus.Front Mol Neurosci. 2019 Apr 18;12:101. doi: 10.3389/fnmol.2019.00101. eCollection 2019. Front Mol Neurosci. 2019. PMID: 31057368 Free PMC article. Review.
-
The Outward Spiral: A vicious cycle model of obesity and cognitive dysfunction.Curr Opin Behav Sci. 2016 Jun;9:40-46. doi: 10.1016/j.cobeha.2015.12.001. Curr Opin Behav Sci. 2016. PMID: 26998507 Free PMC article.
-
Psychological distress and its association with intake of sugar-sweetened beverages, discretionary foods, and alcohol in women during the COVID-19 pandemic in Australia.Nutrition. 2022 Nov-Dec;103-104:111794. doi: 10.1016/j.nut.2022.111794. Epub 2022 Jul 17. Nutrition. 2022. PMID: 36055124 Free PMC article.
References
-
- Acosta GB. A possible interaction between CCKergic and GABAergic systems in the rat brain. Comp Biochem Physiol Toxicol Pharmacol 128: 11–17, 2001 - PubMed
-
- Alzoubi KH, Khabour OF, Salah HA, Abu Rashid BE. The combined effect of sleep deprivation and Western diet on spatial learning and memory: role of BDNF and oxidative stress. J Mol Neurosci 50: 124–133, 2013 - PubMed
-
- Alzoubi KH, Khabour OF, Salah HA, Hasan Z. Vitamin E prevents high-fat high-carbohydrates diet-induced memory impairment: the role of oxidative stress. Physiol Behav 119: 72–78, 2013 - PubMed
-
- Amaral DG, Insausti R, Cowan WM. The entorhinal cortex of the monkey: I. Cytoarchitectonic organization. J Comp Neurol 264: 326–355, 1987 - PubMed
-
- Antin J, Gibbs J, Holt J, Young RC, Smith GP. Cholecystokinin elicits the complete behavioral sequence of satiety in rats. J Comp Physiol Psychol 89: 784–790, 1975 - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

