Glutamate and dopamine transmission from midbrain dopamine neurons share similar release properties but are differentially affected by cocaine
- PMID: 24573277
- PMCID: PMC3935083
- DOI: 10.1523/JNEUROSCI.4958-13.2014
Glutamate and dopamine transmission from midbrain dopamine neurons share similar release properties but are differentially affected by cocaine
Abstract
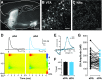
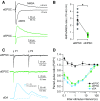
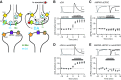
Synaptic transmission between ventral tegmental area and nucleus accumbens (NAc) is critically involved in reward-motivated behaviors and thought to be altered in addiction. In addition to dopamine (DA), glutamate is packaged and released by a subset of mesolimbic DA neurons, eliciting EPSCs onto medium spiny neurons in NAc. Little is known about the properties and modulation of glutamate release from DA midbrain terminals and the effect of cocaine. Using an optogenetic approach to selectively activate midbrain DA fibers, we compared the properties and modulation of DA transients and EPSCs measured using fast-scan cyclic voltammetry and whole-cell recordings in mouse brain slices. DA transients and EPSCs were inhibited by DA receptor D2R agonist and showed a marked paired-pulse depression that required 2 min for full recovery. Cocaine depressed EPSCs amplitude by 50% but enhanced the overall DA transmission from midbrain DA neurons. AMPA and NMDA receptor-mediated EPSCs were equally inhibited by cocaine, suggesting a presynaptic mechanism of action. Pharmacological blockage and genetic deletion of D2R in DA neurons prevented the cocaine-induced inhibition of EPSCs and caused a larger increase in DA transient peak, confirming the involvement of presynaptic D2R. These findings demonstrate that acute cocaine inhibits DA and glutamate release from midbrain DA neurons via presynaptic D2R but has differential overall effects on their transmissions in the NAc. We postulate that cocaine, by blocking DA reuptake, prolongs DA transients and facilitates the feedback inhibition of DA and glutamate release from these terminals.
Keywords: D2 receptors; co-release; cocaine; dopamine; feedback inhibition; glutamate.
Figures



References
-
- Alsiö J, Nordenankar K, Arvidsson E, Birgner C, Mahmoudi S, Halbout B, Smith C, Fortin GM, Olson L, Descarries L, Trudeau LÉ, Kullander K, Lévesque D, Wallén-Mackenzie A. Enhanced sucrose and cocaine self-administration and cue-induced drug seeking after loss of VGLUT2 in midbrain dopamine neurons in mice. J Neurosci. 2011;31:12593–12603. doi: 10.1523/JNEUROSCI.2397-11.2011. - DOI - PMC - PubMed
-
- Bérubé-Carrière N, Guay G, Fortin GM, Kullander K, Olson L, Wallén-Mackenzie Å, Trudeau LE, Descarries L. Ultrastructural characterization of the mesostriatal dopamine innervation in mice, including two mouse lines of conditional VGLUT2 knockout in dopamine neurons. Eur J Neurosci. 2012;35:527–538. doi: 10.1111/j.1460-9568.2012.07992.x. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
