Nonvisual complex spike signals in the rabbit cerebellar flocculus
- PMID: 24573280
- PMCID: PMC3935084
- DOI: 10.1523/JNEUROSCI.3080-13.2014
Nonvisual complex spike signals in the rabbit cerebellar flocculus
Abstract
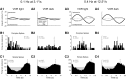
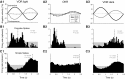
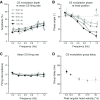
In addition to the well-known signals of retinal image slip, floccular complex spikes (CSs) also convey nonvisual signals. We recorded eye movement and CS activity from Purkinje cells in awake rabbits sinusoidally oscillated in the dark on a vestibular turntable. The stimulus frequency ranged from 0.2 to 1.2 Hz, and the velocity amplitude ranged from 6.3 to 50°/s. The average CS modulation was evaluated at each combination of stimulus frequency and amplitude. More than 75% of the Purkinje cells carried nonvisual CS signals. The amplitude of this modulation remained relatively constant over the entire stimulus range. The phase response of the CS modulation in the dark was opposite to that during the vestibulo-ocular reflex (VOR) in the light. With increased frequency, the phase response systematically shifted from being aligned with contraversive head velocity toward peak contralateral head position. At fixed frequency, the phase response was dependent on peak head velocity, indicating a system nonlinearity. The nonvisual CS modulation apparently reflects a competition between eye movement and vestibular signals, resulting in an eye movement error signal inferred from nonvisual sources. The combination of this error signal with the retinal slip signal in the inferior olive results in a net error signal reporting the discrepancy between the actual visually measured eye movement error and the inferred eye movement error derived from measures of the internal state. The presence of two error signals requires that the role of CSs in models of the floccular control of VOR adaption be expanded beyond retinal slip.
Keywords: Purkinje cell; accessory optic system; climbing fiber; complex spike; inferior olive; prepositus hypoglossi.
Figures







Similar articles
-
Population calcium responses of Purkinje cells in the oculomotor cerebellum driven by nonvisual input.J Neurophysiol. 2021 Oct 1;126(4):1391-1402. doi: 10.1152/jn.00715.2020. Epub 2021 Aug 4. J Neurophysiol. 2021. PMID: 34346783 Free PMC article.
-
Phase relations of Purkinje cells in the rabbit flocculus during compensatory eye movements.J Neurophysiol. 1995 Nov;74(5):2051-64. doi: 10.1152/jn.1995.74.5.2051. J Neurophysiol. 1995. PMID: 8592196
-
Role of cerebellar flocculus in adaptive interaction between optokinetic eye movement response and vestibulo-ocular reflex in pigmented rabbits.Exp Brain Res. 1989;77(3):541-51. doi: 10.1007/BF00249607. Exp Brain Res. 1989. PMID: 2806446
-
Climbing fibers mediate vestibular modulation of both "complex" and "simple spikes" in Purkinje cells.Cerebellum. 2015 Oct;14(5):597-612. doi: 10.1007/s12311-015-0725-1. Cerebellum. 2015. PMID: 26424151 Review.
-
Topsy turvy: functions of climbing and mossy fibers in the vestibulo-cerebellum.Neuroscientist. 2011 Apr;17(2):221-36. doi: 10.1177/1073858410380251. Epub 2011 Feb 28. Neuroscientist. 2011. PMID: 21362689 Free PMC article. Review.
Cited by
-
The Roles of the Olivocerebellar Pathway in Motor Learning and Motor Control. A Consensus Paper.Cerebellum. 2017 Feb;16(1):230-252. doi: 10.1007/s12311-016-0787-8. Cerebellum. 2017. PMID: 27193702 Free PMC article.
-
Population calcium responses of Purkinje cells in the oculomotor cerebellum driven by nonvisual input.J Neurophysiol. 2021 Oct 1;126(4):1391-1402. doi: 10.1152/jn.00715.2020. Epub 2021 Aug 4. J Neurophysiol. 2021. PMID: 34346783 Free PMC article.
-
Climbing fibers predict movement kinematics and performance errors.J Neurophysiol. 2017 Sep 1;118(3):1888-1902. doi: 10.1152/jn.00266.2017. Epub 2017 Jul 12. J Neurophysiol. 2017. PMID: 28701537 Free PMC article.
-
Modeled changes of cerebellar activity in mutant mice are predictive of their learning impairments.Sci Rep. 2016 Nov 2;6:36131. doi: 10.1038/srep36131. Sci Rep. 2016. PMID: 27805050 Free PMC article.
-
Complex Spike Wars: a New Hope.Cerebellum. 2018 Dec;17(6):735-746. doi: 10.1007/s12311-018-0960-3. Cerebellum. 2018. PMID: 29982917 Free PMC article. Review.
References
-
- Albus JS. A theory of cerebellar function. Math Biosci. 1971;10:25–61. doi: 10.1016/0025-5564(71)90051-4. - DOI
-
- Arts MP, De Zeeuw CI, Lips J, Rosbak E, Simpson JI. Effects of nucleus prepositus hypoglossi lesions on visual climbing fiber activity in the rabbit flocculus. J Neurophysiol. 2000;84:2552–2563. - PubMed
-
- Barmack NH, Hess DT. Multiple-unit activity evoked in dorsal cap of inferior olive of the rabbit by visual stimulation. J Neurophysiol. 1980;43:151–164. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
