Functional motifs composed of morphologically homologous neurons repeated in the hindbrain segments
- PMID: 24573288
- PMCID: PMC6795303
- DOI: 10.1523/JNEUROSCI.4610-13.2014
Functional motifs composed of morphologically homologous neurons repeated in the hindbrain segments
Abstract
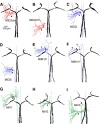
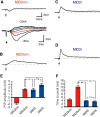
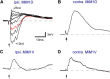
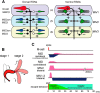
Segmental organization along the neuraxis is a prominent feature of the CNS in vertebrates. In a wide range of fishes, hindbrain segments contain orderly arranged reticulospinal neurons (RSNs). Individual RSNs in goldfish and zebrafish hindbrain are morphologically identified. RSNs sharing similar morphological features are called segmental homologs and repeated in adjacent segments. However, little is known about functional relationships among segmental homologs. Here we investigated the electrophysiological connectivity between the Mauthner cell (M-cell), a pair of giant RSNs in segment 4 (r4) that are known to trigger fast escape behavior, and different series of homologous RSNs in r4-r6. Paired intracellular recordings in adult goldfish revealed unidirectional connections from the M-cell to RSNs. The connectivity was similar in morphological homologs. A single M-cell spike produced IPSPs in dorsally located RSNs (MiD cells) on the ipsilateral side and excitatory postsynaptic depolarization on the contralateral side, except for MiD2cm cells. The inhibitory or excitatory potentials effectively suppressed or enhanced target RSNs spiking, respectively. In contrast to the lateralized effects on MiD cells, single M-cell spiking elicited equally strong depolarizations on bilateral RSNs located ventrally (MiV cells), and the depolarization was high enough for MiV cells to burst. Therefore, the morphological homology of repeated RSNs in r4-r6 and their functional M-cell connectivity were closely correlated, suggesting that each functional connection works as a functional motif during the M-cell-initiated escape.
Keywords: Mauthner cell; escape; hindbrain segments; homologous neurons; reticulospinal neurons; vertebrate.
Figures







References
-
- Barreiro-Iglesias A, Mysiak KS, Adrio F, Rodicio MC, Becker CG, Becker T, Anadón R. Distribution of glycinergic neurons in the brain of glycine transporter-2 Tg (glyt2:gfp) transgenic adult zebrafish: relation with brain-spinal descending systems. J Comp Neurol. 2013;521:389–425. doi: 10.1002/cne.23179. - DOI - PubMed
-
- Bartelmez GW. Mauthner's cell and the nucleus motorius tegmenti. J Comp Neurol. 1915;25:87–128. doi: 10.1002/cne.900250105. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
