Global optogenetic activation of inhibitory interneurons during epileptiform activity
- PMID: 24573293
- PMCID: PMC6795301
- DOI: 10.1523/JNEUROSCI.2734-13.2014
Global optogenetic activation of inhibitory interneurons during epileptiform activity
Abstract
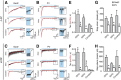
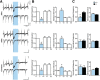
Optogenetic techniques provide powerful tools for bidirectional control of neuronal activity and investigating alterations occurring in excitability disorders, such as epilepsy. In particular, the possibility to specifically activate by light-determined interneuron populations expressing channelrhodopsin-2 provides an unprecedented opportunity of exploring their contribution to physiological and pathological network activity. There are several subclasses of interneurons in cortical areas with different functional connectivity to the principal neurons (e.g., targeting their perisomatic or dendritic compartments). Therefore, one could optogenetically activate specific or a mixed population of interneurons and dissect their selective or concerted inhibitory action on principal cells. We chose to explore a conceptually novel strategy involving simultaneous activation of mixed populations of interneurons by optogenetics and study their impact on ongoing epileptiform activity in mouse acute hippocampal slices. Here we demonstrate that such approach results in a brief initial action potential discharge in CA3 pyramidal neurons, followed by prolonged suppression of ongoing epileptiform activity during light exposure. Such sequence of events was caused by massive light-induced release of GABA from ChR2-expressing interneurons. The inhibition of epileptiform activity was less pronounced if only parvalbumin- or somatostatin-expressing interneurons were activated by light. Our data suggest that global optogenetic activation of mixed interneuron populations is a more effective approach for development of novel therapeutic strategies for epilepsy, but the initial action potential generation in principal neurons needs to be taken in consideration.
Keywords: channelrhodopsin-2; epileptiform activity; hippocampus; interneurons; optogenetics.
Figures


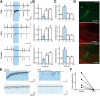
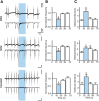



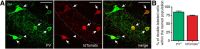
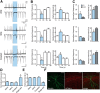

Similar articles
-
Parvalbumin-Positive Inhibitory Interneurons Oppose Propagation But Favor Generation of Focal Epileptiform Activity.J Neurosci. 2015 Jul 1;35(26):9544-57. doi: 10.1523/JNEUROSCI.5117-14.2015. J Neurosci. 2015. PMID: 26134638 Free PMC article.
-
An Optogenetic Approach for Investigation of Excitatory and Inhibitory Network GABA Actions in Mice Expressing Channelrhodopsin-2 in GABAergic Neurons.J Neurosci. 2016 Jun 1;36(22):5961-73. doi: 10.1523/JNEUROSCI.3482-15.2016. J Neurosci. 2016. PMID: 27251618 Free PMC article.
-
Modulation of in vitro epileptiform activity by optogenetic stimulation of parvalbumin-positive interneurons.J Neurophysiol. 2022 Oct 1;128(4):837-846. doi: 10.1152/jn.00192.2022. Epub 2022 Aug 31. J Neurophysiol. 2022. PMID: 36043700
-
Dissecting inhibitory brain circuits with genetically-targeted technologies.Front Neural Circuits. 2014 Oct 17;8:124. doi: 10.3389/fncir.2014.00124. eCollection 2014. Front Neural Circuits. 2014. PMID: 25368555 Free PMC article. Review.
-
Light-activated channels in acute seizures.Epilepsia. 2011 Oct;52 Suppl 8:16-8. doi: 10.1111/j.1528-1167.2011.03226.x. Epilepsia. 2011. PMID: 21967352 Review.
Cited by
-
Microcircuits in Epilepsy: Heterogeneity and Hub Cells in Network Synchronization.Cold Spring Harb Perspect Med. 2015 Nov 2;5(11):a022855. doi: 10.1101/cshperspect.a022855. Cold Spring Harb Perspect Med. 2015. PMID: 26525454 Free PMC article. Review.
-
DREADD in parvalbumin interneurons of the dentate gyrus modulates anxiety, social interaction and memory extinction.Curr Mol Med. 2016;16(1):91-102. doi: 10.2174/1566524016666151222150024. Curr Mol Med. 2016. PMID: 26733123 Free PMC article.
-
Chemogenetic Activation of Feed-Forward Inhibitory Parvalbumin-Expressing Interneurons in the Cortico-Thalamocortical Network During Absence Seizures.Front Cell Neurosci. 2021 May 28;15:688905. doi: 10.3389/fncel.2021.688905. eCollection 2021. Front Cell Neurosci. 2021. PMID: 34122016 Free PMC article.
-
Selective Silencing of Hippocampal Parvalbumin Interneurons Induces Development of Recurrent Spontaneous Limbic Seizures in Mice.J Neurosci. 2017 Aug 23;37(34):8166-8179. doi: 10.1523/JNEUROSCI.3456-16.2017. Epub 2017 Jul 21. J Neurosci. 2017. PMID: 28733354 Free PMC article.
-
Involvement of cortical fast-spiking parvalbumin-positive basket cells in epilepsy.Prog Brain Res. 2016;226:81-126. doi: 10.1016/bs.pbr.2016.04.012. Epub 2016 Jun 7. Prog Brain Res. 2016. PMID: 27323940 Free PMC article. Review.
References
-
- Avoli M. Is epilepsy a disorder of inhibition or excitation? Prog Clin Biol Res. 1983;124:23–37. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous
