Increasing cGMP-dependent protein kinase activity attenuates unilateral ureteral obstruction-induced renal fibrosis
- PMID: 24573388
- PMCID: PMC4010679
- DOI: 10.1152/ajprenal.00657.2013
Increasing cGMP-dependent protein kinase activity attenuates unilateral ureteral obstruction-induced renal fibrosis
Abstract
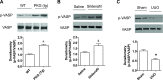
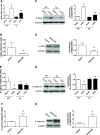
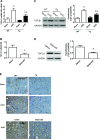
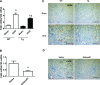
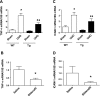
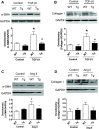
Our previous studies support the protective effect of cGMP and cGMP-dependent protein kinase I (PKG-I) pathway on the development of renal fibrosis. Therefore, in the present studies, we determined whether pharmacologically or genetically increased PKG activity attenuates renal fibrosis in a unilateral ureteral obstruction (UUO) model and also examined the mechanisms involved. To increase PKG activity, we used the phosphodiesterase 5 inhibitor sildenafil and PKG transgenic mice. UUO model was induced in wild-type or PKG-I transgenic mice by ligating the left lateral ureteral and the renal fibrosis was observed after 14 days of ligation. Sildenafil was administered into wild-type UUO mice for 14 days. In vitro, macrophage and proximal tubular cell function was also analyzed. We found that sildenafil treatment or PKG transgenic mice had significantly reduced UUO-induced renal fibrosis, which was associated with reduced TGF-β signaling and reduced macrophage infiltration into kidney interstitial. In vitro data further demonstrated that both macrophages and proximal tubular cells were important sources of UUO-induced renal TGF-β levels. The interaction between macrophages and tubular cells contributes to TGF-β-induced renal fibrosis. Taken together, these data suggest that increasing PKG activity ameliorates renal fibrosis in part through regulation of macrophage and tubular cell function, leading to reduced TGF-β-induced fibrosis.
Keywords: PKG; TGF-β1; UUO; renal fibrosis.
Figures





References
-
- Buvat J, Buttner H, Hatzimouratidis K, Vendeira PA, Moncada I, Boehmer M, Henneges C, Boess FG. Adherence to initial PDE-5 inhibitor treatment: randomized open-label study comparing tadalafil once a day, tadalafil on demand, and sildenafil on demand in patients with erectile dysfunction. J Sex Med 10: 1592–1602, 2013 - PubMed
-
- Chevalier RL, Forbes MS, Thornhill BA. Ureteral obstruction as a model of renal interstitial fibrosis and obstructive nephropathy. Kidney Int 75: 1145–1152, 2009 - PubMed
-
- Diamond JR, Ricardo SD, Klahr S. Mechanisms of interstitial fibrosis in obstructive nephropathy. Semin Nephrol 18: 594–602, 1998 - PubMed
-
- Ding G, Pesek-Diamond I, Diamond JR. Cholesterol, macrophages, and gene expression of TGF-β1 and fibronectin during nephrosis. Am J Physiol Renal Fluid Electrolyte Physiol 264: F577–F584, 1993 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

