The DDN catalytic motif is required for Metnase functions in non-homologous end joining (NHEJ) repair and replication restart
- PMID: 24573677
- PMCID: PMC4036204
- DOI: 10.1074/jbc.M113.533216
The DDN catalytic motif is required for Metnase functions in non-homologous end joining (NHEJ) repair and replication restart
Abstract
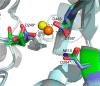
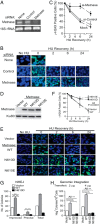
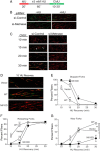
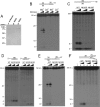
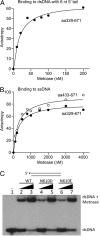
Metnase (or SETMAR) arose from a chimeric fusion of the Hsmar1 transposase downstream of a protein methylase in anthropoid primates. Although the Metnase transposase domain has been largely conserved, its catalytic motif (DDN) differs from the DDD motif of related transposases, which may be important for its role as a DNA repair factor and its enzymatic activities. Here, we show that substitution of DDN(610) with either DDD(610) or DDE(610) significantly reduced in vivo functions of Metnase in NHEJ repair and accelerated restart of replication forks. We next tested whether the DDD or DDE mutants cleave single-strand extensions and flaps in partial duplex DNA and pseudo-Tyr structures that mimic stalled replication forks. Neither substrate is cleaved by the DDD or DDE mutant, under the conditions where wild-type Metnase effectively cleaves ssDNA overhangs. We then characterized the ssDNA-binding activity of the Metnase transposase domain and found that the catalytic domain binds ssDNA but not dsDNA, whereas dsDNA binding activity resides in the helix-turn-helix DNA binding domain. Substitution of Asn-610 with either Asp or Glu within the transposase domain significantly reduces ssDNA binding activity. Collectively, our results suggest that a single mutation DDN(610) → DDD(610), which restores the ancestral catalytic site, results in loss of function in Metnase.
Keywords: DNA Binding Protein; DNA Damage; DNA Enzymes; DNA Repair; DNA Replication.
Figures
Similar articles
-
The SET Domain Is Essential for Metnase Functions in Replication Restart and the 5' End of SS-Overhang Cleavage.PLoS One. 2015 Oct 5;10(10):e0139418. doi: 10.1371/journal.pone.0139418. eCollection 2015. PLoS One. 2015. PMID: 26437079 Free PMC article.
-
Metnase/SETMAR: a domesticated primate transposase that enhances DNA repair, replication, and decatenation.Genetica. 2010 May;138(5):559-66. doi: 10.1007/s10709-010-9452-1. Epub 2010 Mar 23. Genetica. 2010. PMID: 20309721 Free PMC article. Review.
-
Metnase Mediates Loading of Exonuclease 1 onto Single Strand Overhang DNA for End Resection at Stalled Replication Forks.J Biol Chem. 2017 Jan 27;292(4):1414-1425. doi: 10.1074/jbc.M116.745646. Epub 2016 Dec 14. J Biol Chem. 2017. PMID: 27974460 Free PMC article.
-
The human set and transposase domain protein Metnase interacts with DNA Ligase IV and enhances the efficiency and accuracy of non-homologous end-joining.DNA Repair (Amst). 2008 Dec 1;7(12):1927-37. doi: 10.1016/j.dnarep.2008.08.002. Epub 2008 Sep 18. DNA Repair (Amst). 2008. PMID: 18773976 Free PMC article.
-
Structure, Activity, and Function of SETMAR Protein Lysine Methyltransferase.Life (Basel). 2021 Dec 4;11(12):1342. doi: 10.3390/life11121342. Life (Basel). 2021. PMID: 34947873 Free PMC article. Review.
Cited by
-
The DNA repair component Metnase regulates Chk1 stability.Cell Div. 2014 Jul 9;9:1. doi: 10.1186/1747-1028-9-1. eCollection 2014. Cell Div. 2014. PMID: 25024738 Free PMC article.
-
Six domesticated PiggyBac transposases together carry out programmed DNA elimination in Paramecium.Elife. 2018 Sep 18;7:e37927. doi: 10.7554/eLife.37927. Elife. 2018. PMID: 30223944 Free PMC article.
-
Structural and genome-wide analyses suggest that transposon-derived protein SETMAR alters transcription and splicing.J Biol Chem. 2022 May;298(5):101894. doi: 10.1016/j.jbc.2022.101894. Epub 2022 Apr 1. J Biol Chem. 2022. PMID: 35378129 Free PMC article.
-
The roles of the human SETMAR (Metnase) protein in illegitimate DNA recombination and non-homologous end joining repair.DNA Repair (Amst). 2019 Aug;80:26-35. doi: 10.1016/j.dnarep.2019.06.006. Epub 2019 Jun 19. DNA Repair (Amst). 2019. PMID: 31238295 Free PMC article.
-
Paths from DNA damage and signaling to genome rearrangements via homologous recombination.Mutat Res. 2017 Dec;806:64-74. doi: 10.1016/j.mrfmmm.2017.07.008. Epub 2017 Jul 24. Mutat Res. 2017. PMID: 28779875 Free PMC article. Review.
References
-
- Robertson H. M., Zumpano K. L. (1997) Molecular evolution of an ancient mariner transposon, Hsmar1, in the human genome. Gene 205, 203–217 - PubMed
-
- Lee S. H., Oshige M., Durant S. T., Rasila K. K., Williamson E. A., Ramsey H., Kwan L., Nickoloff J. A., Hromas R. (2005) The SET domain protein Metnase mediates foreign DNA integration and links integration to nonhomologous end-joining repair. Proc. Natl. Acad. Sci. U.S.A. 102, 18075–18080 - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

