Mechanistic investigations of unsaturated glucuronyl hydrolase from Clostridium perfringens
- PMID: 24573682
- PMCID: PMC4036275
- DOI: 10.1074/jbc.M113.545293
Mechanistic investigations of unsaturated glucuronyl hydrolase from Clostridium perfringens
Abstract
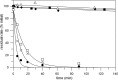
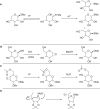
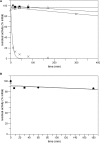
Experiments were carried out to probe the details of the hydration-initiated hydrolysis catalyzed by the Clostridium perfringens unsaturated glucuronyl hydrolase of glycoside hydrolase family 88 in the CAZy classification system. Direct (1)H NMR monitoring of the enzymatic reaction detected no accumulated reaction intermediates in solution, suggesting that rearrangement of the initial hydration product occurs on-enzyme. An attempt at mechanism-based trapping of on-enzyme intermediates using a 1,1-difluoro-substrate was unsuccessful because the probe was too deactivated to be turned over by the enzyme. Kinetic isotope effects arising from deuterium-for-hydrogen substitution at carbons 1 and 4 provide evidence for separate first-irreversible and overall rate-determining steps in the hydration reaction, with two potential mechanisms proposed to explain these results. Based on the positioning of catalytic residues in the enzyme active site, the lack of efficient turnover of a 2-deoxy-2-fluoro-substrate, and several unsuccessful attempts at confirmation of a simpler mechanism involving a covalent glycosyl-enzyme intermediate, the most plausible mechanism is one involving an intermediate bearing an epoxide on carbons 1 and 2.
Keywords: Carbohydrate Metabolism; Chemical Biology; Enzyme; Enzyme Mechanisms; Glycosaminoglycan; Glycosidases; Glycoside Hydrolases; Hydratases; Isotope Effects.
Figures


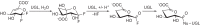


Similar articles
-
Glycoside cleavage by a new mechanism in unsaturated glucuronyl hydrolases.J Am Chem Soc. 2011 Dec 7;133(48):19334-7. doi: 10.1021/ja209067v. Epub 2011 Nov 14. J Am Chem Soc. 2011. PMID: 22047074
-
Mechanistic insights from substrate preference in unsaturated glucuronyl hydrolase.Chembiochem. 2014 Jan 3;15(1):124-34. doi: 10.1002/cbic.201300547. Epub 2013 Nov 13. Chembiochem. 2014. PMID: 24227702
-
A novel unsaturated β-glucuronyl hydrolase involved in ulvan degradation unveils the versatility of stereochemistry requirements in family GH105.J Biol Chem. 2014 Feb 28;289(9):6199-211. doi: 10.1074/jbc.M113.537480. Epub 2014 Jan 9. J Biol Chem. 2014. PMID: 24407291 Free PMC article.
-
Hydration of vinyl ether groups by unsaturated glycoside hydrolases and their role in bacterial pathogenesis.Int Microbiol. 2007 Dec;10(4):233-43. Int Microbiol. 2007. PMID: 18228220 Review.
-
Unusual enzymatic glycoside cleavage mechanisms.Acc Chem Res. 2014 Jan 21;47(1):226-35. doi: 10.1021/ar4001313. Epub 2013 Aug 19. Acc Chem Res. 2014. PMID: 23957528 Review.
Cited by
-
Biochemical Reconstruction of a Metabolic Pathway from a Marine Bacterium Reveals Its Mechanism of Pectin Depolymerization.Appl Environ Microbiol. 2018 Dec 13;85(1):e02114-18. doi: 10.1128/AEM.02114-18. Print 2019 Jan 1. Appl Environ Microbiol. 2018. PMID: 30341080 Free PMC article.
-
Analysis of chondroitin degradation by components of a Bacteroides caccae polysaccharide utilization locus.J Biol Chem. 2025 Jul;301(7):110354. doi: 10.1016/j.jbc.2025.110354. Epub 2025 Jun 7. J Biol Chem. 2025. PMID: 40490139 Free PMC article.
-
GH47 and Other Glycoside Hydrolases Catalyze Glycosidic Bond Cleavage with the Assistance of Substrate Super-arming at the Transition State.ACS Catal. 2021 Aug 20;11:10308-10315. doi: 10.1021/acscatal.1c02750. Epub 2021 Aug 4. ACS Catal. 2021. PMID: 34777906 Free PMC article.
-
An evolutionarily distinct family of polysaccharide lyases removes rhamnose capping of complex arabinogalactan proteins.J Biol Chem. 2017 Aug 11;292(32):13271-13283. doi: 10.1074/jbc.M117.794578. Epub 2017 Jun 21. J Biol Chem. 2017. PMID: 28637865 Free PMC article.
-
Conformational Change in the Active Site of Streptococcal Unsaturated Glucuronyl Hydrolase Through Site-Directed Mutagenesis at Asp-115.Protein J. 2016 Aug;35(4):300-9. doi: 10.1007/s10930-016-9673-y. Protein J. 2016. PMID: 27402448
References
-
- Ernst S., Langer R., Cooney C. L., Sasisekharan R. (1995) Enzymatic degradation of glycosaminoglycans. Crit. Rev. Biochem. Mol. Biol. 30, 387–444 - PubMed
-
- Jackson R. L., Busch S. J., Cardin A. D. (1991) Glycosaminoglycans–Molecular properties, protein interactions, and role in physiological processes. Physiol. Rev. 71, 481–539 - PubMed
-
- Hashimoto W., Kobayashi E., Nankai H., Sato N., Miya T., Kawai S., Murata K. (1999) Unsaturated glucuronyl hydrolase of Bacillus sp. GL1: Novel enzyme prerequisite for metabolism of unsaturated oligosaccharides produced by polysaccharide lyases. Arch. Biochem. Biophys. 368, 367–374 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

