The presence of multiple cellular defects associated with a novel G50E iron-sulfur cluster scaffold protein (ISCU) mutation leads to development of mitochondrial myopathy
- PMID: 24573684
- PMCID: PMC4036159
- DOI: 10.1074/jbc.M113.526665
The presence of multiple cellular defects associated with a novel G50E iron-sulfur cluster scaffold protein (ISCU) mutation leads to development of mitochondrial myopathy
Abstract
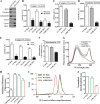
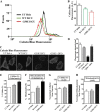
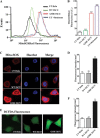
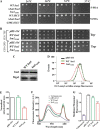
Iron-sulfur (Fe-S) clusters are versatile cofactors involved in regulating multiple physiological activities, including energy generation through cellular respiration. Initially, the Fe-S clusters are assembled on a conserved scaffold protein, iron-sulfur cluster scaffold protein (ISCU), in coordination with iron and sulfur donor proteins in human mitochondria. Loss of ISCU function leads to myopathy, characterized by muscle wasting and cardiac hypertrophy. In addition to the homozygous ISCU mutation (g.7044G→C), compound heterozygous patients with severe myopathy have been identified to carry the c.149G→A missense mutation converting the glycine 50 residue to glutamate. However, the physiological defects and molecular mechanism associated with G50E mutation have not been elucidated. In this report, we uncover mechanistic insights concerning how the G50E ISCU mutation in humans leads to the development of severe ISCU myopathy, using a human cell line and yeast as the model systems. The biochemical results highlight that the G50E mutation results in compromised interaction with the sulfur donor NFS1 and the J-protein HSCB, thus impairing the rate of Fe-S cluster synthesis. As a result, electron transport chain complexes show significant reduction in their redox properties, leading to loss of cellular respiration. Furthermore, the G50E mutant mitochondria display enhancement in iron level and reactive oxygen species, thereby causing oxidative stress leading to impairment in the mitochondrial functions. Thus, our findings provide compelling evidence that the respiration defect due to impaired biogenesis of Fe-S clusters in myopathy patients leads to manifestation of complex clinical symptoms.
Keywords: Electron Transport System (ETS); Hsp70; Iron-Sulfur Cluster; Iron-Sulfur Protein; J-protein; Mitochondrial Diseases; Molecular Chaperone; Myopathy; Reactive Oxygen Species (ROS).
Figures




Similar articles
-
Mapping Key Residues of ISD11 Critical for NFS1-ISD11 Subcomplex Stability: IMPLICATIONS IN THE DEVELOPMENT OF MITOCHONDRIAL DISORDER, COXPD19.J Biol Chem. 2015 Oct 23;290(43):25876-90. doi: 10.1074/jbc.M115.678508. Epub 2015 Sep 4. J Biol Chem. 2015. PMID: 26342079 Free PMC article.
-
Clinical manifestation and a new ISCU mutation in iron-sulphur cluster deficiency myopathy.Brain. 2009 Aug;132(Pt 8):2170-9. doi: 10.1093/brain/awp152. Epub 2009 Jun 30. Brain. 2009. PMID: 19567699
-
Solution structure of the iron-sulfur cluster cochaperone HscB and its binding surface for the iron-sulfur assembly scaffold protein IscU.Biochemistry. 2008 Sep 9;47(36):9394-404. doi: 10.1021/bi800502r. Epub 2008 Aug 15. Biochemistry. 2008. PMID: 18702525 Free PMC article.
-
Molecular chaperones HscA/Ssq1 and HscB/Jac1 and their roles in iron-sulfur protein maturation.Crit Rev Biochem Mol Biol. 2007 Mar-Apr;42(2):95-111. doi: 10.1080/10409230701322298. Crit Rev Biochem Mol Biol. 2007. PMID: 17453917 Review.
-
Iron-sulfur cluster biogenesis in mammalian cells: New insights into the molecular mechanisms of cluster delivery.Biochim Biophys Acta. 2015 Jun;1853(6):1493-512. doi: 10.1016/j.bbamcr.2014.09.009. Epub 2014 Sep 19. Biochim Biophys Acta. 2015. PMID: 25245479 Free PMC article. Review.
Cited by
-
Robust glyoxalase activity of Hsp31, a ThiJ/DJ-1/PfpI family member protein, is critical for oxidative stress resistance in Saccharomyces cerevisiae.J Biol Chem. 2015 Oct 30;290(44):26491-507. doi: 10.1074/jbc.M115.673624. Epub 2015 Sep 14. J Biol Chem. 2015. PMID: 26370081 Free PMC article.
-
The Eukaryotic-Specific ISD11 Is a Complex-Orphan Protein with Ability to Bind the Prokaryotic IscS.PLoS One. 2016 Jul 18;11(7):e0157895. doi: 10.1371/journal.pone.0157895. eCollection 2016. PLoS One. 2016. PMID: 27427956 Free PMC article.
-
Stress-induced proteins enhance thermal endurance in soil bacterium Priestia aryabhattai strain PSK.N2.Antonie Van Leeuwenhoek. 2025 Jul 2;118(8):102. doi: 10.1007/s10482-025-02118-4. Antonie Van Leeuwenhoek. 2025. PMID: 40601179
-
A Highly Conserved Iron-Sulfur Cluster Assembly Machinery between Humans and Amoeba Dictyostelium discoideum: The Characterization of Frataxin.Int J Mol Sci. 2020 Sep 17;21(18):6821. doi: 10.3390/ijms21186821. Int J Mol Sci. 2020. PMID: 32957566 Free PMC article.
-
FDX2 and ISCU Gene Variations Lead to Rhabdomyolysis With Distinct Severity and Iron Regulation.Neurol Genet. 2022 Jan 19;8(1):e648. doi: 10.1212/NXG.0000000000000648. eCollection 2022 Feb. Neurol Genet. 2022. PMID: 35079622 Free PMC article.
References
-
- Craig E. A., Voisine C., Schilke B. (1999) Mitochondrial iron metabolism in the yeast Saccharomyces cerevisiae. Biol. Chem. 380, 1167–1173 - PubMed
-
- Lill R. (2009) Function and biogenesis of iron-sulphur proteins. Nature 460, 831–838 - PubMed
-
- Meyer J. (2008) Iron-sulfur protein folds, iron-sulfur chemistry, and evolution. J. Biol. Inorg. Chem. 13, 157–170 - PubMed
-
- Hinchliffe P., Sazanov L. A. (2005) Organization of iron-sulfur clusters in respiratory complex I. Science 309, 771–774 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

