ZEB2-transgene expression in the epidermis compromises the integrity of the epidermal barrier through the repression of different tight junction proteins
- PMID: 24573695
- PMCID: PMC11113794
- DOI: 10.1007/s00018-014-1589-0
ZEB2-transgene expression in the epidermis compromises the integrity of the epidermal barrier through the repression of different tight junction proteins
Abstract
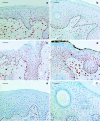
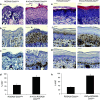
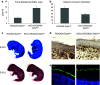
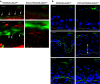
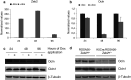
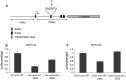
Epithelial homeostasis within the epidermis is maintained by means of multiple cell-cell adhesion complexes such as adherens junctions, tight junctions, gap junctions, and desmosomes. These complexes co-operate in the formation and the regulation of the epidermal barrier. Disruption of the epidermal barrier through the deregulation of the above complexes is the cause behind a number of skin disorders such as psoriasis, dermatitis, keratosis, and others. During epithelial-to-mesenchymal transition (EMT), epithelial cells lose their adhesive capacities and gain mesenchymal properties. ZEB transcription factors are key inducers of EMT. In order to gain a better understanding of the functional role of ZEB2 in epidermal homeostasis, we generated a mouse model with conditional overexpression of Zeb2 in the epidermis. Our analysis revealed that Zeb2 expression in the epidermis leads to hyperproliferation due to the combined downregulation of different tight junction proteins compromising the epidermal barrier. Using two epidermis-specific in vivo models and in vitro promoter assays, we identified occludin as a new Zeb2 target gene. Immunohistological analysis performed on human skin biopsies covering various pathogeneses revealed ZEB2 expression in the epidermis of pemphigus vulgaris. Collectively, our data support the notion for a potential role of ZEB2 in intracellular signaling of this disease.
Conflict of interest statement
The authors declare that they have no conflicts of interest.
Figures
Similar articles
-
Epidermal tight junctions: ZO-1 and occludin are expressed in mature, developing, and affected skin and in vitro differentiating keratinocytes.J Invest Dermatol. 2001 Nov;117(5):1050-8. doi: 10.1046/j.0022-202x.2001.01493.x. J Invest Dermatol. 2001. PMID: 11710912
-
Fra-1/AP-1 induces EMT in mammary epithelial cells by modulating Zeb1/2 and TGFβ expression.Cell Death Differ. 2015 Feb;22(2):336-50. doi: 10.1038/cdd.2014.157. Epub 2014 Oct 10. Cell Death Differ. 2015. PMID: 25301070 Free PMC article.
-
TFAP2A is a component of the ZEB1/2 network that regulates TGFB1-induced epithelial to mesenchymal transition.Biol Direct. 2017 Apr 17;12(1):8. doi: 10.1186/s13062-017-0180-7. Biol Direct. 2017. PMID: 28412966 Free PMC article.
-
Role of ZEB Family Members in Proliferation, Metastasis, and Chemoresistance of Prostate Cancer Cells: Revealing Signaling Networks.Curr Cancer Drug Targets. 2021;21(9):749-767. doi: 10.2174/1568009621666210601114631. Curr Cancer Drug Targets. 2021. PMID: 34077345 Review.
-
Zeb2: A multifunctional regulator of nervous system development.Prog Neurobiol. 2015 Sep;132:81-95. doi: 10.1016/j.pneurobio.2015.07.001. Epub 2015 Jul 18. Prog Neurobiol. 2015. PMID: 26193487 Review.
Cited by
-
Dysregulation of the Skin-Liver Axis in Prurigo Nodularis: An Integrated Genomic, Transcriptomic, and Population-Based Analysis.Genes (Basel). 2024 Jan 23;15(2):146. doi: 10.3390/genes15020146. Genes (Basel). 2024. PMID: 38397136 Free PMC article.
-
Dedicator of Cytokinesis 5 Regulates Keratinocyte Function and Promotes Diabetic Wound Healing.Diabetes. 2021 May;70(5):1170-1184. doi: 10.2337/db20-1008. Epub 2021 Feb 24. Diabetes. 2021. PMID: 33627322 Free PMC article.
-
MITF in melanoma: mechanisms behind its expression and activity.Cell Mol Life Sci. 2015 Apr;72(7):1249-60. doi: 10.1007/s00018-014-1791-0. Epub 2014 Nov 30. Cell Mol Life Sci. 2015. PMID: 25433395 Free PMC article. Review.
-
Scaffolding proteins in the development and maintenance of the epidermal permeability barrier.Tissue Barriers. 2017 Oct 2;5(4):e1341969. doi: 10.1080/21688370.2017.1341969. Epub 2017 Jun 30. Tissue Barriers. 2017. PMID: 28665776 Free PMC article. Review.
-
ZEB2 drives intra-tumor heterogeneity and skin squamous cell carcinoma formation with distinct EMP transition states.iScience. 2024 Oct 16;27(11):111169. doi: 10.1016/j.isci.2024.111169. eCollection 2024 Nov 15. iScience. 2024. PMID: 39555401 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

