The delayed-release combination of doxylamine and pyridoxine (Diclegis®/Diclectin ®) for the treatment of nausea and vomiting of pregnancy
- PMID: 24574047
- PMCID: PMC4030125
- DOI: 10.1007/s40272-014-0065-5
The delayed-release combination of doxylamine and pyridoxine (Diclegis®/Diclectin ®) for the treatment of nausea and vomiting of pregnancy
Abstract
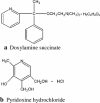
Nausea and vomiting of pregnancy (NVP) affects up to 85 % of all pregnancies. Effective treatment can greatly improve a woman's quality of life, reduce the risk for maternal and fetal complications, and reduce healthcare costs. Unfortunately, many women receive either no pharmacological treatment or are recommended therapies for which fetal safety and efficacy have not been established. First-line treatment of NVP, as recommended by several leading healthcare and professional organizations, is the combination of doxylamine and pyridoxine. This combination, formulated as a 10 mg/10 mg delayed-release tablet, was approved by the US Food and Drug Administration (FDA) for the treatment of NVP in April 2013 under the brand name Diclegis(®), and has been on the Canadian market since 1979, currently under the brand name Diclectin(®). The efficacy of Diclegis(®)/Diclectin(®) has been demonstrated in several clinical trials, and, more importantly, studies on more than 200,000 women exposed to doxylamine and pyridoxine in the first trimester of pregnancy have demonstrated no increased fetal risk for congenital malformations and other adverse pregnancy outcomes. The present review aims to present the scientific evidence on the effectiveness and fetal safety of Diclegis(®)/Diclectin(®) for the treatment of NVP to justify its use as first-line treatment for NVP.
Figures
Similar articles
-
Demonstration of early efficacy results of the delayed-release combination of doxylamine-pyridoxine for the treatment of nausea and vomiting of pregnancy.BMC Pregnancy Childbirth. 2016 Nov 24;16(1):371. doi: 10.1186/s12884-016-1172-9. BMC Pregnancy Childbirth. 2016. PMID: 27881103 Free PMC article. Clinical Trial.
-
Maternal safety of the delayed-release doxylamine and pyridoxine combination for nausea and vomiting of pregnancy; a randomized placebo controlled trial.BMC Pregnancy Childbirth. 2015 Mar 18;15:59. doi: 10.1186/s12884-015-0488-1. BMC Pregnancy Childbirth. 2015. PMID: 25884778 Free PMC article. Clinical Trial.
-
Doxylamine/pyridoxine for nausea and vomiting in pregnancy.Drug Ther Bull. 2019 Mar;57(3):38-41. doi: 10.1136/dtb.2018.000053. Epub 2019 Jan 31. Drug Ther Bull. 2019. PMID: 30705026 Review. No abstract available.
-
Diclegis for nausea and vomiting of pregnancy.Med Lett Drugs Ther. 2013 Aug 5;55(1422):61-2. Med Lett Drugs Ther. 2013. PMID: 23917384 No abstract available.
-
A new pharmacologic treatment for nausea and vomiting of pregnancy.Nurs Womens Health. 2014 Feb-Mar;18(1):73-77. doi: 10.1111/1751-486X.12096. Nurs Womens Health. 2014. PMID: 24548499 Review.
Cited by
-
Diagnosis and treatment of hyperemesis gravidarum.CMAJ. 2024 Apr 14;196(14):E477-E485. doi: 10.1503/cmaj.221502. CMAJ. 2024. PMID: 38621783 Free PMC article. Review. No abstract available.
-
Nausea and Vomiting of Pregnancy and its Management with the Dual-Release Formulation of Doxylamine and Pyridoxine.Geburtshilfe Frauenheilkd. 2024 Feb 8;84(2):144-152. doi: 10.1055/a-2225-5883. eCollection 2024 Feb. Geburtshilfe Frauenheilkd. 2024. PMID: 38344043 Free PMC article.
-
8-Way Randomized Controlled Trial of Doxylamine, Pyridoxine and Dicyclomine for Nausea and Vomiting during Pregnancy: Restoration of Unpublished Information.PLoS One. 2017 Jan 4;12(1):e0167609. doi: 10.1371/journal.pone.0167609. eCollection 2017. PLoS One. 2017. PMID: 28052111 Free PMC article. Clinical Trial.
-
Nausea and vomiting of pregnancy - What's new?Auton Neurosci. 2017 Jan;202:62-72. doi: 10.1016/j.autneu.2016.05.002. Epub 2016 May 13. Auton Neurosci. 2017. PMID: 27209471 Free PMC article. Review.
-
Suboptimal maternal and cord plasma pyridoxal 5' phosphate concentrations are uncommon in a cohort of Canadian pregnant women and newborn infants.Matern Child Nutr. 2018 Jan;14(1):e12467. doi: 10.1111/mcn.12467. Epub 2017 May 24. Matern Child Nutr. 2018. PMID: 28544455 Free PMC article.
References
-
- Lacroix R, Eason E, Melzack R. Nausea and vomiting during pregnancy: a prospective study of its frequency, intensity, and patterns of change. Am J Obstet Gynecol. 2000;182(4):931–7. - PubMed
-
- APGO. Nausea and vomiting of pregnancy. APGO Educational series on women’s health issues. Boston: Jespersen & Associates, LLC; 2013.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

