Phosphorylation of α-tubulin by protein kinase C stimulates microtubule dynamics in human breast cells
- PMID: 24574051
- PMCID: PMC4113324
- DOI: 10.1002/cm.21167
Phosphorylation of α-tubulin by protein kinase C stimulates microtubule dynamics in human breast cells
Abstract
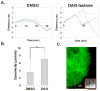
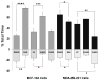
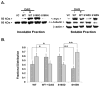
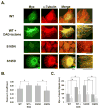
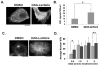
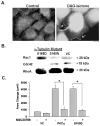
Protein kinase C (PKC) engenders motility through phosphorylation of α-tubulin at Ser-165 in nontransformed MCF-10A cells. Live cell imaging explored the impact of PKC-mediated phosphorylation on microtubule (MT) dynamics. MTs fluorescently labeled with GFP-α-tubulin were treated with diacylglycerol (DAG)-lactone (a membrane-permeable PKC activator), or cotransfected with a pseudophosphorylated S165D-α6-tubulin mutant. Each condition increased the dynamicity of MTs by stimulating the rate and duration of the growth phase and decreasing the frequency of catastrophe. In MDA-MB-231 metastatic breast cells where the intrinsic PKC activity is high, these MT growth parameters were also high but could be suppressed by expression of phosphorylation-resistant S165N-α6-tubulin or by treatment with a pan-PKC inhibitor (bis-indoleylmaleimide). Subcellular fractionation and immunofluorescence of MCF-10A cells showed that phosphorylation (via DAG-lactone) or pseudophosphorylation of α6-tubulin increased its partitioning into MTs as compared to controls, and produced longer, more stable MTs. Following expression of the plus-end binding protein GFP-EB1, DAG-lactone accelerated the formation and increased the number of nascent MTs. Expression of S165D-α6-tubulin promoted Rac1 activation and Rac1-dependent cell motility. These findings call attention to PKC-mediated phosphorylation of α-tubulin as a novel mechanism for controlling the dynamics of MTs that result in cell movement.
Keywords: Rac1; live cell imaging; motility; mutant; partitioning.
Copyright © 2014 Wiley Periodicals, Inc.
Figures
Similar articles
-
Phosphorylation of alpha6-tubulin by protein kinase Calpha activates motility of human breast cells.J Biol Chem. 2009 Jun 26;284(26):17648-56. doi: 10.1074/jbc.M902005200. Epub 2009 Apr 29. J Biol Chem. 2009. PMID: 19406749 Free PMC article.
-
Phosphomimetic Mutation at Ser165 of α-Tubulin Promotes the Persistence of GTP Caps in Microtubules.Biochemistry. 2022 Jul 19;61(14):1508-1516. doi: 10.1021/acs.biochem.2c00154. Epub 2022 Jul 7. Biochemistry. 2022. PMID: 35799350
-
Phosphorylation state of Ser165 in α-tubulin is a toggle switch that controls proliferating human breast tumors.Cell Signal. 2018 Dec;52:74-82. doi: 10.1016/j.cellsig.2018.08.021. Epub 2018 Sep 1. Cell Signal. 2018. PMID: 30176291 Free PMC article.
-
Phosphorylation of Cdc42 effector protein-4 (CEP4) by protein kinase C promotes motility of human breast cells.J Biol Chem. 2014 Sep 12;289(37):25844-54. doi: 10.1074/jbc.M114.577783. Epub 2014 Aug 1. J Biol Chem. 2014. PMID: 25086031 Free PMC article.
-
Intrinsic and Extrinsic Factors Affecting Microtubule Dynamics in Normal and Cancer Cells.Molecules. 2020 Aug 14;25(16):3705. doi: 10.3390/molecules25163705. Molecules. 2020. PMID: 32823874 Free PMC article. Review.
Cited by
-
The Effect of SIN1 and Microtubules on Insulin Induced PKC ζ Activation.Med Sci Monit. 2017 Jul 28;23:3666-3672. doi: 10.12659/msm.905555. Med Sci Monit. 2017. PMID: 28751630 Free PMC article.
-
Posttranslational modifications of the cytoskeleton.Cytoskeleton (Hoboken). 2021 Apr;78(4):142-173. doi: 10.1002/cm.21679. Epub 2021 Jul 2. Cytoskeleton (Hoboken). 2021. PMID: 34152688 Free PMC article. Review.
-
Tubulin Post-Translational Modifications and Microtubule Dynamics.Int J Mol Sci. 2017 Oct 21;18(10):2207. doi: 10.3390/ijms18102207. Int J Mol Sci. 2017. PMID: 29065455 Free PMC article. Review.
-
Evaluation of active Rac1 levels in cancer cells: A case of misleading conclusions from immunofluorescence analysis.J Biol Chem. 2020 Oct 2;295(40):13698-13710. doi: 10.1074/jbc.RA120.013919. Epub 2020 Aug 14. J Biol Chem. 2020. PMID: 32817335 Free PMC article.
-
The Tubulin Code in Microtubule Dynamics and Information Encoding.Dev Cell. 2020 Jul 6;54(1):7-20. doi: 10.1016/j.devcel.2020.06.008. Dev Cell. 2020. PMID: 32634400 Free PMC article. Review.
References
-
- Abeyweera TP, Rotenberg SA. Design and characterization of a traceable protein kinase Cα. Biochemistry. 2007;46:2364–2370. - PubMed
-
- Banyard J, Anand-Apte B, Symons M, Zetter BR. Motility and invasion are differentially modulated by Rho family GTPases. Oncogene. 2000;19:580–591. - PubMed
-
- Bolte S, Cordelieres F. A guided tour into subcellular colocalization analysis in light microscopy. J Microscopy. 2006;224:213–232. - PubMed
-
- Chan A, Andreae PM, Northcote PT, Miller JH. Peloruside A inhibits microtubule dynamics in a breast cancer cell line MCF7. Invest New Drugs. 2011;29:615–626. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

