Heat shock protein 70 enhances mucosal immunity against human norovirus when coexpressed from a vesicular stomatitis virus vector
- PMID: 24574391
- PMCID: PMC3993811
- DOI: 10.1128/JVI.00019-14
Heat shock protein 70 enhances mucosal immunity against human norovirus when coexpressed from a vesicular stomatitis virus vector
Abstract
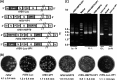
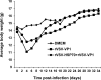
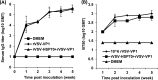
Human norovirus (NoV) accounts for 95% of nonbacterial gastroenteritis worldwide. Currently, there is no vaccine available to combat human NoV as it is not cultivable and lacks a small-animal model. Recently, we demonstrated that recombinant vesicular stomatitis virus (rVSV) expressing human NoV capsid protein (rVSV-VP1) induced strong immunities in mice (Y. Ma and J. Li, J. Virol. 85:2942-2952, 2011). To further improve the safety and efficacy of the vaccine candidate, heat shock protein 70 (HSP70) was inserted into the rVSV-VP1 backbone vector. A second construct was generated in which the firefly luciferase (Luc) gene was inserted in place of HSP70 as a control for the double insertion. The resultant recombinant viruses (rVSV-HSP70-VP1 and rVSV-Luc-VP1) were significantly more attenuated in cell culture and viral spread in mice than rVSV-VP1. At the inoculation dose of 1.0 × 10(6) PFU, rVSV-HSP70-VP1 triggered significantly higher vaginal IgA than rVSV-VP1 and significantly higher fecal and vaginal IgA responses than rVSV-Luc-VP1, although serum IgG and T cell responses were similar. At the inoculation dose of 5.0 × 10(6) PFU, rVSV-HSP70-VP1 stimulated significantly higher T cell, fecal, and vaginal IgA responses than rVSV-VP1. Fecal and vaginal IgA responses were also significantly increased when combined vaccination of rVSV-VP1 and rVSV-HSP70 was used. Collectively, these data indicate that (i) insertion of an additional gene (HSP70 or Luc) into the rVSV-VP1 backbone further attenuates the VSV-based vaccine in vitro and in vivo, thus improving the safety of the vaccine candidate, and (ii) HSP70 enhances the human NoV-specific mucosal and T cell immunities triggered by a VSV-based human NoV vaccine.
Importance: Human norovirus (NoV) is responsible for more than 95% of acute nonbacterial gastroenteritis worldwide. Currently, there is no vaccine for this virus. Development of a live attenuated vaccine for human NoV has not been possible because it is uncultivable. Thus, a live vector-based vaccine may provide an alternative vaccine strategy. In this study, we developed a vesicular stomatitis virus (VSV)-based human NoV vaccine candidate. We constructed rVSV-HSP70-VP1, coexpressing heat shock protein (HSP70) and capsid (VP1) genes of human NoV, and rVSV-Luc-VP1, coexpressing firefly luciferase (Luc) and VP1 genes. We found that VSVs with a double gene insertion were significantly more attenuated than VSV with a single VP1 insertion (rVSV-VP1). Furthermore, we found that coexpression or coadministration of HSP70 from VSV vector significantly enhanced human NoV-specific mucosal immunity. Collectively, we developed an improved live vectored vaccine candidate for human NoV which will be useful for future clinical studies.
Figures






Similar articles
-
Vesicular stomatitis virus as a vector to deliver virus-like particles of human norovirus: a new vaccine candidate against an important noncultivable virus.J Virol. 2011 Mar;85(6):2942-52. doi: 10.1128/JVI.02332-10. Epub 2011 Jan 12. J Virol. 2011. PMID: 21228240 Free PMC article.
-
Replication-deficient Sendai virus expressing human norovirus capsid protein elicits robust NoV-specific antibody and T-cell responses in mice.Microbes Infect. 2025 Feb;27(2):105412. doi: 10.1016/j.micinf.2024.105412. Epub 2024 Sep 3. Microbes Infect. 2025. PMID: 39236991
-
Newcastle disease virus vector producing human norovirus-like particles induces serum, cellular, and mucosal immune responses in mice.J Virol. 2014 Sep 1;88(17):9718-27. doi: 10.1128/JVI.01570-14. Epub 2014 Jun 11. J Virol. 2014. Retraction in: J Virol. 2020 Feb 28;94(6):e01869-19. doi: 10.1128/JVI.01869-19. PMID: 24920815 Free PMC article. Retracted.
-
Live virus vaccines based on a vesicular stomatitis virus (VSV) backbone: Standardized template with key considerations for a risk/benefit assessment.Vaccine. 2016 Dec 12;34(51):6597-6609. doi: 10.1016/j.vaccine.2016.06.071. Epub 2016 Jul 6. Vaccine. 2016. PMID: 27395563 Free PMC article. Review.
-
A Brighton Collaboration standardized template with key considerations for a benefit/risk assessment for the emergent vesicular stomatitis virus (VSV) viral vector vaccine for Lassa fever.Vaccine. 2025 Jun 11;58:127137. doi: 10.1016/j.vaccine.2025.127137. Epub 2025 May 13. Vaccine. 2025. PMID: 40367816 Review.
Cited by
-
Identification of a Region in the Common Amino-terminal Domain of Hendra Virus P, V, and W Proteins Responsible for Phase Transition and Amyloid Formation.Biomolecules. 2021 Sep 7;11(9):1324. doi: 10.3390/biom11091324. Biomolecules. 2021. PMID: 34572537 Free PMC article.
-
Norovirus vaccines: Correlates of protection, challenges and limitations.Hum Vaccin Immunother. 2016 Jul 2;12(7):1653-69. doi: 10.1080/21645515.2015.1125054. Epub 2016 Feb 2. Hum Vaccin Immunother. 2016. PMID: 26836766 Free PMC article. Review.
-
Coexpression of respiratory syncytial virus (RSV) fusion (F) protein and attachment glycoprotein (G) in a vesicular stomatitis virus (VSV) vector system provides synergistic effects against RSV infection in a cotton rat model.Vaccine. 2021 Nov 16;39(47):6817-6828. doi: 10.1016/j.vaccine.2021.10.042. Epub 2021 Oct 23. Vaccine. 2021. PMID: 34702618 Free PMC article.
-
Norovirus vaccines and potential antinorovirus drugs: recent advances and future perspectives.Future Virol. 2015;10(7):899-913. doi: 10.2217/fvl.15.57. Future Virol. 2015. PMID: 26568768 Free PMC article.
-
Mucosal Delivery of Recombinant Vesicular Stomatitis Virus Vectors Expressing Envelope Proteins of Respiratory Syncytial Virus Induces Protective Immunity in Cotton Rats.J Virol. 2021 Feb 24;95(6):e02345-20. doi: 10.1128/JVI.02345-20. Print 2021 Feb 24. J Virol. 2021. PMID: 33408176 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

