Yeast virus-derived stimulator of the innate immune system augments the efficacy of virus vector-based immunotherapy
- PMID: 24574403
- PMCID: PMC4019141
- DOI: 10.1128/JVI.03819-13
Yeast virus-derived stimulator of the innate immune system augments the efficacy of virus vector-based immunotherapy
Abstract
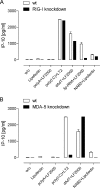
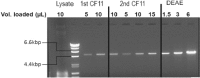
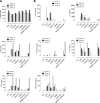
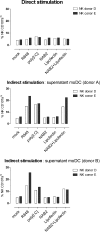
To identify novel stimulators of the innate immune system, we constructed a panel of eight HEK293 cell lines double positive for human Toll-like receptors (TLRs) and an NF-κB-inducible reporter gene. Screening of a large variety of compounds and cellular extracts detected a TLR3-activating compound in a microsomal yeast extract. Fractionation of this extract identified an RNA molecule of 4.6 kb, named nucleic acid band 2 (NAB2), that was sufficient to confer the activation of TLR3. Digests with single- and double-strand-specific RNases showed the double-strand nature of this RNA, and its sequence was found to be identical to that of the genome of the double-stranded RNA (dsRNA) L-BC virus of Saccharomyces cerevisiae. A large-scale process of production and purification of this RNA was established on the basis of chemical cell lysis and dsRNA-specific chromatography. NAB2 complexed with the cationic lipid Lipofectin but neither NAB2 nor Lipofectin alone induced the secretion of interleukin-12(p70) [IL-12(p70)], alpha interferon, gamma interferon-induced protein 10, macrophage inflammatory protein 1β, or IL-6 in human monocyte-derived dendritic cells. While NAB2 activated TLR3, Lipofectin-stabilized NAB2 also signaled via the cytoplasmic sensor for RNA recognition MDA-5. A significant increase of RMA-MUC1 tumor rejection and survival was observed in C57BL/6 mice after prophylactic vaccination with MUC1-encoding modified vaccinia virus Ankara (MVA) and NAB2-Lipofectin. This combination of immunotherapies strongly increased at the injection sites the percentage of infiltrating natural killer (NK) cells and plasmacytoid dendritic cells (pDCs), cell types which can modulate innate and adaptive immune responses.
Importance: Virus-based cancer vaccines offer a good alternative to the treatment of cancer but could be improved. Starting from a screening approach, we have identified and characterized an unexplored biological molecule with immunomodulatory characteristics which augments the efficacy of an MVA-based immunotherapeutic agent. The immune modulator consists of the purified dsRNA genome isolated from a commercially used yeast strain, NAB2, mixed with a cationic lipid, Lipofectin. NAB2-Lipofectin stimulates the immune system via TLR3 and MDA-5. When it was injected at the MVA vaccination site, the immune modulator increased survival in a preclinical tumor model. We could demonstrate that NAB2-Lipofectin augments the MVA-induced infiltration of natural killer and plasmacytoid dendritic cells. We suggest indirect mechanisms of activation of these cell types by the influence of NAB2-Lipofectin on innate and adaptive immunity. Detailed analysis of cell migration at the vaccine injection site and the appropriate choice of an immune modulator should be considered to achieve the rational improvement of virus vector-based vaccination by immune modulators.
Figures





Similar articles
-
Recombinant modified vaccinia virus Ankara generating excess early double-stranded RNA transiently activates protein kinase R and triggers enhanced innate immune responses.J Virol. 2014 Dec;88(24):14396-411. doi: 10.1128/JVI.02082-14. Epub 2014 Oct 8. J Virol. 2014. PMID: 25297997 Free PMC article.
-
Innate immune responses in human monocyte-derived dendritic cells are highly dependent on the size and the 5' phosphorylation of RNA molecules.J Immunol. 2011 Aug 15;187(4):1713-21. doi: 10.4049/jimmunol.1100361. Epub 2011 Jul 8. J Immunol. 2011. PMID: 21742966
-
Sequential administration of a MVA-based MUC1 cancer vaccine and the TLR9 ligand Litenimod (Li28) improves local immune defense against tumors.Vaccine. 2017 Jan 23;35(4):577-585. doi: 10.1016/j.vaccine.2016.12.020. Epub 2016 Dec 21. Vaccine. 2017. PMID: 28012777
-
Particulate formulations for the delivery of poly(I:C) as vaccine adjuvant.Adv Drug Deliv Rev. 2013 Oct;65(10):1386-99. doi: 10.1016/j.addr.2013.05.013. Epub 2013 Jun 7. Adv Drug Deliv Rev. 2013. PMID: 23751781 Review.
-
Beyond dsRNA: Toll-like receptor 3 signalling in RNA-induced immune responses.Biochem J. 2014 Mar 1;458(2):195-201. doi: 10.1042/BJ20131492. Biochem J. 2014. PMID: 24524192 Review.
Cited by
-
Unc-13 homolog D mediates an antiviral effect of the chromosome 19 microRNA cluster miR-517a.J Cell Sci. 2020 Nov 19;134(5):jcs246769. doi: 10.1242/jcs.246769. J Cell Sci. 2020. PMID: 33093239 Free PMC article.
-
Enhancing Efficacy and Stability of an Antiheroin Vaccine: Examination of Antinociception, Opioid Binding Profile, and Lethality.Mol Pharm. 2018 Mar 5;15(3):1062-1072. doi: 10.1021/acs.molpharmaceut.7b00933. Epub 2018 Feb 19. Mol Pharm. 2018. PMID: 29420901 Free PMC article.
-
The Dual Role of the Innate Immune System in the Effectiveness of mRNA Therapeutics.Int J Mol Sci. 2023 Oct 1;24(19):14820. doi: 10.3390/ijms241914820. Int J Mol Sci. 2023. PMID: 37834268 Free PMC article. Review.
-
Heterogeneity and efficacy of immunotherapy in multiple cancer: insights from a meta-analysis.Biol Proced Online. 2025 May 20;27(1):17. doi: 10.1186/s12575-025-00274-5. Biol Proced Online. 2025. PMID: 40389815 Free PMC article.
-
A Novel Virus Alters Gene Expression and Vacuolar Morphology in Malassezia Cells and Induces a TLR3-Mediated Inflammatory Immune Response.mBio. 2020 Sep 1;11(5):e01521-20. doi: 10.1128/mBio.01521-20. mBio. 2020. PMID: 32873759 Free PMC article.
References
-
- Mayr A, Stickl H, Muller HK, Danner K, Singer H. 1978. The smallpox vaccination strain MVA: marker, genetic structure, experience gained with the parenteral vaccination and behavior in organisms with a debilitated defence mechanism. Zentralbl. Bakteriol. B 167:375–390 (Authors' translation.) - PubMed
-
- Bansal AS, Bruce J, Devine PL, Scells B, Zimmermann PV. 1997. Serum cytokines and tumour markers in patients with non-small cell carcinoma of the lung. Dis. Markers 13:195–199 - PubMed
-
- Quoix E, Ramlau R, Westeel V, Papai Z, Madroszyk A, Riviere A, Koralewski P, Breton JL, Stoelben E, Braun D, Debieuvre D, Lena H, Buyse M, Chenard MP, Acres B, Lacoste G, Bastien B, Tavernaro A, Bizouarne N, Bonnefoy JY, Limacher JM. 2011. Therapeutic vaccination with TG4010 and first-line chemotherapy in advanced non-small-cell lung cancer: a controlled phase 2B trial. Lancet Oncol. 12:1125–1133. 10.1016/S1470-2045(11)70259-5 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

