Human herpesvirus 6A infection in CD46 transgenic mice: viral persistence in the brain and increased production of proinflammatory chemokines via Toll-like receptor 9
- PMID: 24574405
- PMCID: PMC4019085
- DOI: 10.1128/JVI.03763-13
Human herpesvirus 6A infection in CD46 transgenic mice: viral persistence in the brain and increased production of proinflammatory chemokines via Toll-like receptor 9
Abstract
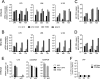
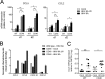
Human herpesvirus 6 (HHV-6) is widely spread in the human population and has been associated with several neuroinflammatory diseases, including multiple sclerosis. To develop a small-animal model of HHV-6 infection, we analyzed the susceptibility of several lines of transgenic mice expressing human CD46, identified as a receptor for HHV-6. We showed that HHV-6A (GS) infection results in the expression of viral transcripts in primary brain glial cultures from CD46-expressing mice, while HHV-6B (Z29) infection was inefficient. HHV-6A DNA persisted for up to 9 months in the brain of CD46-expressing mice but not in the nontransgenic littermates, whereas HHV-6B DNA levels decreased rapidly after infection in all mice. Persistence in the brain was observed with infectious but not heat-inactivated HHV-6A. Immunohistological studies revealed the presence of infiltrating lymphocytes in periventricular areas of the brain of HHV-6A-infected mice. Furthermore, HHV-6A stimulated the production of a panel of proinflammatory chemokines in primary brain glial cultures, including CCL2, CCL5, and CXCL10, and induced the expression of CCL5 in the brains of HHV-6A-infected mice. HHV-6A-induced production of chemokines in the primary glial cultures was dependent on the stimulation of toll-like receptor 9 (TLR9). Finally, HHV-6A induced signaling through human TLR9 as well, extending observations from the murine model to human infection. Altogether, this study presents a first murine model for HHV-6A-induced brain infection and suggests a role for TLR9 in the HHV-6A-initiated production of proinflammatory chemokines in the brain, opening novel perspectives for the study of virus-associated neuropathology.
Importance: HHV-6 infection has been related to neuroinflammatory diseases; however, the lack of a suitable small-animal infection model has considerably hampered further studies of HHV-6-induced neuropathogenesis. In this study, we have characterized a new model for HHV-6 infection in mice expressing the human CD46 protein. Infection of CD46 transgenic mice with HHV-6A resulted in long-term persistence of viral DNA in the brains of infected animals and was followed by lymphocyte infiltration and upregulation of the CCL5 chemokine in the absence of clinical signs of disease. The secretion of a panel of chemokines was increased after infection in primary murine brain glial cultures, and the HHV-6-induced chemokine expression was inhibited when TLR9 signaling was blocked. These results describe the first murine model for HHV-6A-induced brain infection and suggest the importance of the TLR9 pathway in HHV-6A-initiated neuroinflammation.
Figures





Similar articles
-
Induction of Proinflammatory Multiple Sclerosis-Associated Retrovirus Envelope Protein by Human Herpesvirus-6A and CD46 Receptor Engagement.Front Immunol. 2018 Dec 6;9:2803. doi: 10.3389/fimmu.2018.02803. eCollection 2018. Front Immunol. 2018. PMID: 30574140 Free PMC article.
-
CD46 Isoforms Influence the Mode of Entry by Human Herpesvirus 6A/B in T Cells.J Virol. 2022 Mar 9;96(5):e0155721. doi: 10.1128/JVI.01557-21. Epub 2022 Jan 12. J Virol. 2022. PMID: 35019717 Free PMC article.
-
Recent developments in animal models for human herpesvirus 6A and 6B.Curr Opin Virol. 2014 Dec;9:97-103. doi: 10.1016/j.coviro.2014.09.012. Epub 2014 Oct 14. Curr Opin Virol. 2014. PMID: 25462440 Free PMC article. Review.
-
Novel marmoset (Callithrix jacchus) model of human Herpesvirus 6A and 6B infections: immunologic, virologic and radiologic characterization.PLoS Pathog. 2013 Jan;9(1):e1003138. doi: 10.1371/journal.ppat.1003138. Epub 2013 Jan 31. PLoS Pathog. 2013. PMID: 23382677 Free PMC article.
-
Roseolovirus-associated encephalitis in immunocompetent and immunocompromised individuals.J Neurovirol. 2017 Feb;23(1):1-19. doi: 10.1007/s13365-016-0473-0. Epub 2016 Aug 18. J Neurovirol. 2017. PMID: 27538995 Free PMC article. Review.
Cited by
-
Human Herpesvirus-6 and -7 in the Brain Microenvironment of Persons with Neurological Pathology and Healthy People.Int J Mol Sci. 2021 Feb 27;22(5):2364. doi: 10.3390/ijms22052364. Int J Mol Sci. 2021. PMID: 33673426 Free PMC article.
-
Herpesvirus Infection of Endothelial Cells as a Systemic Pathological Axis in Myalgic Encephalomyelitis/Chronic Fatigue Syndrome.Viruses. 2024 Apr 8;16(4):572. doi: 10.3390/v16040572. Viruses. 2024. PMID: 38675914 Free PMC article. Review.
-
Active HHV-6 Infection of Cerebellar Purkinje Cells in Mood Disorders.Front Microbiol. 2018 Aug 21;9:1955. doi: 10.3389/fmicb.2018.01955. eCollection 2018. Front Microbiol. 2018. PMID: 30186267 Free PMC article.
-
Comparative Analysis of Roseoloviruses in Humans, Pigs, Mice, and Other Species.Viruses. 2019 Nov 30;11(12):1108. doi: 10.3390/v11121108. Viruses. 2019. PMID: 31801268 Free PMC article. Review.
-
Marmosets as models of infectious diseases.Front Cell Infect Microbiol. 2024 Feb 23;14:1340017. doi: 10.3389/fcimb.2024.1340017. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 38465237 Free PMC article. Review.
References
-
- Ablashi D, Agut H, Alvarez-Lafuente R, Clark DA, Dewhurst S, Diluca D, Flamand L, Frenkel N, Gallo R, Gompels UA, Höllsberg P, Jacobson S, Luppi M, Lusso P, Malnati M, Medveczky P, Mori Y, Pellett PE, Pritchett JC, Yamanishi K, Yoshikawa T. 6 November 2013. Classification of HHV-6A and HHV-6B as distinct viruses. Arch. Virol. 10.1007/s00705-013-1902-5 - DOI - PMC - PubMed
-
- Yamanishi K, Okuno T, Shiraki K, Takahashi M, Kondo T, Asano Y, Kurata T. 1988. Identification of human herpesvirus-6 as a causal agent for exanthem subitum. Lancet i:1065–1067 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

