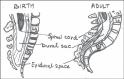
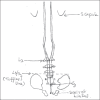
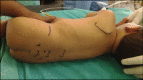
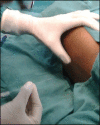
Spinal anesthesia in children: A review
- PMID: 24574586
- PMCID: PMC3927267
- DOI: 10.4103/0970-9185.125687
Spinal anesthesia in children: A review
Abstract
Even after a vast safety record, the role of spinal anesthesia (SA) as a primary anesthetic technique in children remains contentious and is mainly limited to specialized pediatric centers. It is usually practiced on moribund former preterm infants (<60 weeks post-conception) to reduce the incidence of post-operative apnea when compared to general anesthesia (GA). However, there is ample literature to suggest its safety and efficacy for suitable procedures in older children as well. SA in children has many advantages as in adults with an added advantage of minimal cardio-respiratory disturbance. Recently, several reports from animal studies have raised serious concerns regarding the harmful effects of GA on young developing brain. This may further increase the utility of SA in children as it provides all components of balanced anesthesia technique. Also, SA can be an economical option for countries with finite resources. Limited duration of surgical anesthesia in children is one of the major deterrents for its widespread use in them. To overcome this, several additives like epinephrine, clonidine, fentanyl, morphine, neostigmine etc. have been used and found to be effective even in neonates. But, the developing spinal cord may also be vulnerable to drug-related toxicity, though this has not been systematically evaluated in children. So, adjuvants and drugs with widest therapeutic index should be preferred in children. Despite its widespread use, incidence of side-effects is low and permanent neurological sequalae have not been reported with SA. Literature yields encouraging results regarding its safety and efficacy. Technical skills and constant vigilance of experienced anesthesia providers is indispensable to achieve good results with this technique.
Keywords: Additives; children; complications; pediatric; regional anesthesia; spinal; spinal anesthesia.
Conflict of interest statement
Figures
References
-
- Bier A. Experiment regarding the cocainization of the spinal cord. Zentralbl Chir. 1899;51:361–9.
-
- Bainbridge WS. A report of twelve operations on infants and young children during spinal anesthesia. Arch Pediatr. 1901;18:570–4.
-
- Gray HT. A study of subarachnoid block in children and infants. Lancet. 1909;2:913–7.
-
- Berkowitz S, Greene BA. Spinal anaesthesia in children: Report based on 350 patients under 13 years. Anesthesiology. 1951;12:376–87. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources