Deletion of Gpr128 results in weight loss and increased intestinal contraction frequency
- PMID: 24574718
- PMCID: PMC3923024
- DOI: 10.3748/wjg.v20.i2.498
Deletion of Gpr128 results in weight loss and increased intestinal contraction frequency
Abstract
Aim: To generate a Gpr128 gene knockout mouse model and to investigate its phenotypes and the biological function of the Gpr128 gene.
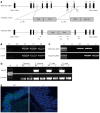
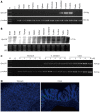
Methods: Bacterial artificial chromosome-retrieval methods were used for constructing the targeting vector. Using homologous recombination and microinjection technology, a Gpr128 knockout mouse model on a mixed 129/BL6 background was generated. The mice were genotyped by polymerase chain reaction (PCR) analysis of tail DNA and fed a standard laboratory chow diet. Animals of both sexes were used, and the phenotypes were assessed by histological, biochemical, molecular and physiological analyses. Semi-quantitative reverse transcription-PCR and Northern blotting were used to determine the tissue distribution of Gpr128 mRNA. Beginning at the age of 4 wk, body weights were recorded every 4 wk. Food, feces, blood and organ samples were collected to analyze food consumption, fecal quantity, organ weight and constituents of the blood and plasma. A Trendelenburg preparation was utilized to examine intestinal motility in wild-type (WT) and Gpr128(-/-) mice at the age of 8 and 32 wk.
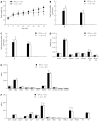
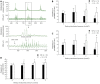
Results: Gpr128 mRNA was highly and exclusively detected in the intestinal tissues. Targeted deletion of Gpr128 in adult mice resulted in reduced body weight gain, and mutant mice exhibited an increased frequency of peristaltic contraction and slow wave potential of the small intestine. The Gpr128(+/+) mice gained more weight on average than the Gpr128(-/-) mice since 24 wk, being 30.81 ± 2.84 g and 25.74 ± 4.50 g, respectively (n = 10, P < 0.01). The frequency of small intestinal peristaltic contraction was increased in Gpr128(-/-) mice. At the age of 8 wk, the frequency of peristalsis with an intraluminal pressure of 3 cmH2O was 6.6 ± 2.3 peristalsis/15 min in Gpr128(-/-) intestine (n = 5) vs 2.6 ± 1.7 peristalsis/15 min in WT intestine (n = 5, P < 0.05). At the age of 32 wk, the frequency of peristaltic contraction with an intraluminal pressure of 2 and 3 cmH2O was 4.6 ± 2.3 and 3.1 ± 0.8 peristalsis/15 min in WT mice (n = 8), whereas in Gpr128(-/-) mice (n = 8) the frequency of contraction was 8.3 ± 3.0 and 7.4 ± 3.1 peristalsis/15 min, respectively (2 cmH2O: P < 0.05 vs WT; 3 cmH2O: P < 0.01 vs WT). The frequency of slow wave potential in Gpr128(-/-) intestine (35.8 ± 4.3, 36.4 ± 4.2 and 37.1 ± 4.8/min with an intraluminal pressure of 1, 2 and 3 cmH2O, n = 8) was also higher than in WT intestine (30.6 ± 4.2, 31.4 ± 3.9 and 31.9 ± 4.5/min, n = 8, P < 0.05).
Conclusion: We have generated a mouse model with a targeted deletion of Gpr128 and found reduced body weight and increased intestinal contraction frequency in this animal model.
Keywords: G-protein-coupled receptors; Gpr128; Intestinal contraction frequency; Knockout mouse; Weight loss.
Figures
References
-
- Lander ES, Linton LM, Birren B, Nusbaum C, Zody MC, Baldwin J, Devon K, Dewar K, Doyle M, FitzHugh W, et al. Initial sequencing and analysis of the human genome. Nature. 2001;409:860–921. - PubMed
-
- Venter JC, Adams MD, Myers EW, Li PW, Mural RJ, Sutton GG, Smith HO, Yandell M, Evans CA, Holt RA, Gocayne JD, Amanatides P, Ballew RM, Huson DH, Wortman JR, Zhang Q, Kodira CD, Zheng XH, Chen L, Skupski M, Subramanian G, Thomas PD, Zhang J, Gabor Miklos GL, Nelson C, Broder S, Clark AG, Nadeau J, McKusick VA, Zinder N, Levine AJ, Roberts RJ, Simon M, Slayman C, Hunkapiller M, Bolanos R, Delcher A, Dew I, Fasulo D, Flanigan M, Florea L, Halpern A, Hannenhalli S, Kravitz S, Levy S, Mobarry C, Reinert K, Remington K, Abu-Threideh J, Beasley E, Biddick K, Bonazzi V, Brandon R, Cargill M, Chandramouliswaran I, Charlab R, Chaturvedi K, Deng Z, Di Francesco V, Dunn P, Eilbeck K, Evangelista C, Gabrielian AE, Gan W, Ge W, Gong F, Gu Z, Guan P, Heiman TJ, Higgins ME, Ji RR, Ke Z, Ketchum KA, Lai Z, Lei Y, Li Z, Li J, Liang Y, Lin X, Lu F, Merkulov GV, Milshina N, Moore HM, Naik AK, Narayan VA, Neelam B, Nusskern D, Rusch DB, Salzberg S, Shao W, Shue B, Sun J, Wang Z, Wang A, Wang X, Wang J, Wei M, Wides R, Xiao C, Yan C, Yao A, Ye J, Zhan M, Zhang W, Zhang H, Zhao Q, Zheng L, Zhong F, Zhong W, Zhu S, Zhao S, Gilbert D, Baumhueter S, Spier G, Carter C, Cravchik A, Woodage T, Ali F, An H, Awe A, Baldwin D, Baden H, Barnstead M, Barrow I, Beeson K, Busam D, Carver A, Center A, Cheng ML, Curry L, Danaher S, Davenport L, Desilets R, Dietz S, Dodson K, Doup L, Ferriera S, Garg N, Gluecksmann A, Hart B, Haynes J, Haynes C, Heiner C, Hladun S, Hostin D, Houck J, Howland T, Ibegwam C, Johnson J, Kalush F, Kline L, Koduru S, Love A, Mann F, May D, McCawley S, McIntosh T, McMullen I, Moy M, Moy L, Murphy B, Nelson K, Pfannkoch C, Pratts E, Puri V, Qureshi H, Reardon M, Rodriguez R, Rogers YH, Romblad D, Ruhfel B, Scott R, Sitter C, Smallwood M, Stewart E, Strong R, Suh E, Thomas R, Tint NN, Tse S, Vech C, Wang G, Wetter J, Williams S, Williams M, Windsor S, Winn-Deen E, Wolfe K, Zaveri J, Zaveri K, Abril JF, Guigó R, Campbell MJ, Sjolander KV, Karlak B, Kejariwal A, Mi H, Lazareva B, Hatton T, Narechania A, Diemer K, Muruganujan A, Guo N, Sato S, Bafna V, Istrail S, Lippert R, Schwartz R, Walenz B, Yooseph S, Allen D, Basu A, Baxendale J, Blick L, Caminha M, Carnes-Stine J, Caulk P, Chiang YH, Coyne M, Dahlke C, Mays A, Dombroski M, Donnelly M, Ely D, Esparham S, Fosler C, Gire H, Glanowski S, Glasser K, Glodek A, Gorokhov M, Graham K, Gropman B, Harris M, Heil J, Henderson S, Hoover J, Jennings D, Jordan C, Jordan J, Kasha J, Kagan L, Kraft C, Levitsky A, Lewis M, Liu X, Lopez J, Ma D, Majoros W, McDaniel J, Murphy S, Newman M, Nguyen T, Nguyen N, Nodell M, Pan S, Peck J, Peterson M, Rowe W, Sanders R, Scott J, Simpson M, Smith T, Sprague A, Stockwell T, Turner R, Venter E, Wang M, Wen M, Wu D, Wu M, Xia A, Zandieh A, Zhu X. The sequence of the human genome. Science. 2001;291:1304–1351. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

