Predictors of survival in patients with established cirrhosis and hepatocellular carcinoma treated with sorafenib
- PMID: 24574751
- PMCID: PMC3921487
- DOI: 10.3748/wjg.v20.i3.786
Predictors of survival in patients with established cirrhosis and hepatocellular carcinoma treated with sorafenib
Abstract
Aim: To investigate in greater detail the efficacy and safety of sorafenib for the treatment of hepatocellular carcinoma (HCC) in patients with established cirrhosis.
Methods: From October 2009 to July 2012 patients with an established diagnosis of cirrhosis and HCC treated with sorafenib were consecutively enrolled. According to the Barcelona Clinic Liver Cancer (BCLC) classification, patients were in the advanced stage (BCLC-C) or in the intermediate stage (BCLC-B) but unfit or unresponsive to other therapeutic strategies. Treatment was evaluated performing a 4-phase computed tomography or magnetic resonance imaging scan every 2-3 mo, and analyzed according to the modified Response Evaluation Criteria in Solid Tumors. Sorafenib was administered at 800 mg/d, until radiological progression or occurrence of unacceptable adverse events (AEs). Univariate and multivariate analyses identified predictors of 16-wk clinical benefit and overall survival.
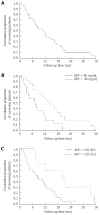
Results: Forty-four patients were enrolled, 15 had intermediate HCC and 14 a Child-Pugh score of B7. AEs caused treatment interruption in 19 patients (43%), and median treatment duration was shorter in this subset (5 wk vs 19 wk, P < 0.001) and in the BCLC-C subgroup (13 wk vs 40 wk, P = 0.015). No significant differences in the reason for treatment interruption or in treatment duration were found comparing patients in Child-Pugh class A vs B or in patients older or younger than 70 years. After 16 wk of treatment, 18 patients (41%) had stable disease or partial response. Patients with viral infection or BCLC-C were at higher risk of disease progression. ECOG, extrahepatic spread, macrovascular invasion, alpha-fetoprotein or alkaline phosphatase levels at admission were independent predictors of overall survival.
Conclusion: In patients with cirrhosis and HCC treated with sorafenib, AEs are a common cause of early treatment withdrawal. Vascular invasion and extrahepatic spread condition early response to treatment and survival. Baseline biochemical parameters may be helpful to identify patients at higher risk of shorter overall survival.
Keywords: Adverse events; Barcelona Clinic Liver Cancer; Cirrhosis; Hepatocellular carcinoma; Sorafenib.
Figures
Similar articles
-
Indicators of sorafenib efficacy in patients with advanced hepatocellular carcinoma.World J Gastroenterol. 2014 Sep 21;20(35):12581-7. doi: 10.3748/wjg.v20.i35.12581. World J Gastroenterol. 2014. PMID: 25253961 Free PMC article.
-
Clinical parameters predictive of outcomes in sorafenib-treated patients with advanced hepatocellular carcinoma.Liver Int. 2013 Jul;33(6):950-7. doi: 10.1111/liv.12168. Epub 2013 Apr 21. Liver Int. 2013. PMID: 23601249
-
Sorafenib for the treatment of advanced hepatocellular carcinoma with extrahepatic metastasis: a prospective multicenter cohort study.Cancer Med. 2015 Dec;4(12):1836-43. doi: 10.1002/cam4.548. Epub 2015 Oct 16. Cancer Med. 2015. PMID: 26471348 Free PMC article.
-
Sorafenib: from literature to clinical practice.Ann Oncol. 2013 Apr;24 Suppl 2:ii30-7. doi: 10.1093/annonc/mdt055. Ann Oncol. 2013. PMID: 23715941 Review.
-
Treatment of hepatocellular carcinoma combining sorafenib and transarterial locoregional therapy: state of the science.J Vasc Interv Radiol. 2013 Aug;24(8):1123-34. doi: 10.1016/j.jvir.2013.01.494. Epub 2013 Apr 4. J Vasc Interv Radiol. 2013. PMID: 23562168 Review.
Cited by
-
Prognostic Value of VEGF in Hepatocellular Carcinoma Patients Treated with Sorafenib: A Meta-Analysis.Med Sci Monit. 2015 Oct 18;21:3144-51. doi: 10.12659/msm.894617. Med Sci Monit. 2015. PMID: 26476711 Free PMC article.
-
Advanced hepatocellular carcinoma and sorafenib: Diagnosis, indications, clinical and radiological follow-up.World J Hepatol. 2015 May 18;7(8):1041-53. doi: 10.4254/wjh.v7.i8.1041. World J Hepatol. 2015. PMID: 26052393 Free PMC article. Review.
-
Effect and safety of sorafenib in patients with intermediate hepatocellular carcinoma who received transarterial chemoembolization: A retrospective comparative study.World J Clin Cases. 2018 May 16;6(5):74-83. doi: 10.12998/wjcc.v6.i5.74. World J Clin Cases. 2018. PMID: 29774219 Free PMC article.
-
Evaluation of Fruquintinib in the Continuum of Care of Patients with Colorectal Cancer.Int J Mol Sci. 2023 Mar 19;24(6):5840. doi: 10.3390/ijms24065840. Int J Mol Sci. 2023. PMID: 36982913 Free PMC article. Review.
-
The prognostic factors between different viral etiologies among advanced hepatocellular carcinoma patients receiving sorafenib treatment.Kaohsiung J Med Sci. 2019 Oct;35(10):624-632. doi: 10.1002/kjm2.12105. Epub 2019 Jun 28. Kaohsiung J Med Sci. 2019. PMID: 31254328 Free PMC article.
References
-
- El-Serag HB. Hepatocellular carcinoma. N Engl J Med. 2011;365:1118–1127. - PubMed
-
- European Association For The Study Of The Liver, European Organisation For Research And Treatment Of Cancer. EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. - PubMed
-
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, Blanc JF, de Oliveira AC, Santoro A, Raoul JL, Forner A, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359:378–390. - PubMed
-
- Cheng AL, Kang YK, Chen Z, Tsao CJ, Qin S, Kim JS, Luo R, Feng J, Ye S, Yang TS, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10:25–34. - PubMed
-
- Cammà C, Cabibbo G, Petta S, Enea M, Iavarone M, Grieco A, Gasbarrini A, Villa E, Zavaglia C, Bruno R, et al. Cost-effectiveness of sorafenib treatment in field practice for patients with hepatocellular carcinoma. Hepatology. 2013;57:1046–1054. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

