Colorectal cancer biomarkers: to be or not to be? Cautionary tales from a road well travelled
- PMID: 24574763
- PMCID: PMC3921542
- DOI: 10.3748/wjg.v20.i4.888
Colorectal cancer biomarkers: to be or not to be? Cautionary tales from a road well travelled
Abstract
Colorectal cancer (CRC) is the second most common cause of cancer-related death worldwide and places a major economic burden on the global health care system. The time frame for development from premalignant to malignant disease typically spans 10-15 years, and this latent period provides an ideal opportunity for early detection and intervention to improve patient outcomes. Currently, early diagnosis of CRC is hampered by a lack of suitable non-invasive biomarkers that are clinically or economically acceptable for population-based screening. New blood-based protein biomarkers for early detection of CRC are therefore urgently required. The success of clinical biomarker discovery and validation studies is critically dependent on understanding and adjusting for potential experimental, analytical, and biological factors that can interfere with the robust interpretation of results. In this review we outline some important considerations for research groups undertaking biomarker research with exemplars from our studies. Implementation of experimental strategies to minimise the potential effects of these problems will facilitate the identification of panels of biomarkers with the sensitivity and specificity required for the development of successful tests for the early detection and surveillance of CRC.
Keywords: Bias; Biomarker; Colorectal cancer; Diagnostic; Discovery; Validation.
Figures

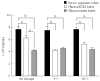
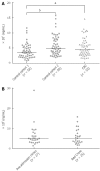
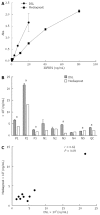
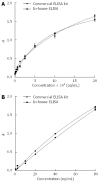
References
-
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. GLOBOCAN 2008 v1.2, Cancer Incidence and Mortality Worldwide: IARC CancerBase No. 10 [Internet] Lyon, France: International Agency for Research on Cancer; 2010.
-
- Washington, DC: AICR; 2007. Food, nutrition, physical activity, and the prevention of cancer: a global perspective.
-
- Etzioni R, Urban N, Ramsey S, McIntosh M, Schwartz S, Reid B, Radich J, Anderson G, Hartwell L. The case for early detection. Nat Rev Cancer. 2003;3:243–252. - PubMed
-
- Kuipers EJ, Rösch T, Bretthauer M. Colorectal cancer screening--optimizing current strategies and new directions. Nat Rev Clin Oncol. 2013;10:130–142. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

