Neuroanatomy of lower gastrointestinal pain disorders
- PMID: 24574773
- PMCID: PMC3921524
- DOI: 10.3748/wjg.v20.i4.1005
Neuroanatomy of lower gastrointestinal pain disorders
Abstract
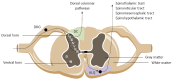
Chronic abdominal pain accompanying intestinal inflammation emerges from the hyperresponsiveness of neuronal, immune and endocrine signaling pathways within the intestines, the peripheral and the central nervous system. In this article we review how the sensory nerve information from the healthy and the hypersensitive bowel is encoded and conveyed to the brain. The gut milieu is continuously monitored by intrinsic enteric afferents, and an extrinsic nervous network comprising vagal, pelvic and splanchnic afferents. The extrinsic afferents convey gut stimuli to second order neurons within the superficial spinal cord layers. These neurons cross the white commissure and ascend in the anterolateral quadrant and in the ipsilateral dorsal column of the dorsal horn to higher brain centers, mostly subserving regulatory functions. Within the supraspinal regions and the brainstem, pathways descend to modulate the sensory input. Because of this multiple level control, only a small proportion of gut signals actually reaches the level of consciousness to induce sensation or pain. In inflammatory bowel disease (IBD) and irritable bowel syndrome (IBS) patients, however, long-term neuroplastic changes have occurred in the brain-gut axis which results in chronic abdominal pain. This sensitization may be driven on the one hand by peripheral mechanisms within the intestinal wall which encompasses an interplay between immunocytes, enterochromaffin cells, resident macrophages, neurons and smooth muscles. On the other hand, neuronal synaptic changes along with increased neurotransmitter release in the spinal cord and brain leads to a state of central wind-up. Also life factors such as but not limited to inflammation and stress contribute to hypersensitivity. All together, the degree to which each of these mechanisms contribute to hypersensitivity in IBD and IBS might be disease- and even patient-dependent. Mapping of sensitization throughout animal and human studies may significantly improve our understanding of sensitization in IBD and IBS. On the long run, this knowledge can be put forward in potential therapeutic targets for abdominal pain in these conditions.
Keywords: Afferent nerves; Chronic pain; Inflammatory bowel disease; Irritable bowel syndrome; Sensitisation; Sensory nerves; Visceral hypersensitivity.
Figures

Similar articles
-
Abdominal pain in Irritable Bowel Syndrome: a review of putative psychological, neural and neuro-immune mechanisms.Brain Behav Immun. 2011 Mar;25(3):386-94. doi: 10.1016/j.bbi.2010.11.010. Epub 2010 Nov 20. Brain Behav Immun. 2011. PMID: 21094682 Review.
-
Visceral pain: spinal afferents, enteric mast cells, enteric nervous system and stress.Curr Pharm Des. 2011;17(16):1573-5. doi: 10.2174/138161211796196918. Curr Pharm Des. 2011. PMID: 21548869 Review.
-
Intestinal Fungal Dysbiosis Is Associated With Visceral Hypersensitivity in Patients With Irritable Bowel Syndrome and Rats.Gastroenterology. 2017 Oct;153(4):1026-1039. doi: 10.1053/j.gastro.2017.06.004. Epub 2017 Jun 15. Gastroenterology. 2017. PMID: 28624575
-
Irritable bowel syndrome and visceral hypersensitivity : risk factors and pathophysiological mechanisms.Acta Gastroenterol Belg. 2016 Mar;79(1):29-38. Acta Gastroenterol Belg. 2016. PMID: 26852761 Review.
-
Brain and gut interactions in irritable bowel syndrome: new paradigms and new understandings.Curr Gastroenterol Rep. 2014 Apr;16(4):379. doi: 10.1007/s11894-014-0379-z. Curr Gastroenterol Rep. 2014. PMID: 24595616 Free PMC article. Review.
Cited by
-
Glutamatergic Signaling Along The Microbiota-Gut-Brain Axis.Int J Mol Sci. 2019 Mar 25;20(6):1482. doi: 10.3390/ijms20061482. Int J Mol Sci. 2019. PMID: 30934533 Free PMC article. Review.
-
Beyond Pain Relief: A Review on Cannabidiol Potential in Medical Therapies.Pharmaceuticals (Basel). 2023 Jan 20;16(2):155. doi: 10.3390/ph16020155. Pharmaceuticals (Basel). 2023. PMID: 37259306 Free PMC article. Review.
-
Visceral hypersensitivity in inflammatory bowel diseases and irritable bowel syndrome: The role of proteases.World J Gastroenterol. 2016 Dec 21;22(47):10275-10286. doi: 10.3748/wjg.v22.i47.10275. World J Gastroenterol. 2016. PMID: 28058009 Free PMC article. Review.
-
Progressive impairment in gastric and duodenal slow waves and autonomic function during progression of type 2 diabetes in rats.Am J Physiol Gastrointest Liver Physiol. 2025 Apr 1;328(4):G386-G398. doi: 10.1152/ajpgi.00278.2024. Epub 2025 Feb 24. Am J Physiol Gastrointest Liver Physiol. 2025. PMID: 39993032 Free PMC article.
-
An intravital window to image the colon in real time.Nat Commun. 2019 Dec 11;10(1):5647. doi: 10.1038/s41467-019-13699-w. Nat Commun. 2019. PMID: 31827103 Free PMC article.
References
-
- Wagtmans MJ, Verspaget HW, Lamers CB, van Hogezand RA. Crohn’s disease in the elderly: a comparison with young adults. J Clin Gastroenterol. 1998;27:129–133. - PubMed
-
- Geboes K, Collins S. Structural abnormalities of the nervous system in Crohn’s disease and ulcerative colitis. Neurogastroenterol Motil. 1998;10:189–202. - PubMed
-
- De Schepper HU, De Man JG, Moreels TG, Pelckmans PA, De Winter BY. Review article: gastrointestinal sensory and motor disturbances in inflammatory bowel disease - clinical relevance and pathophysiological mechanisms. Aliment Pharmacol Ther. 2008;27:621–637. - PubMed
-
- Cross RK, Wilson KT, Binion DG. Narcotic use in patients with Crohn’s disease. Am J Gastroenterol. 2005;100:2225–2229. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

