Renal adverse effects of sunitinib and its clinical significance: a single-center experience in Korea
- PMID: 24574832
- PMCID: PMC3932394
- DOI: 10.3904/kjim.2014.29.1.40
Renal adverse effects of sunitinib and its clinical significance: a single-center experience in Korea
Abstract
Background/aims: Sunitinib is an oral multitargeted tyrosine kinase inhibitor used mainly for the treatment of metastatic renal cell carcinoma. The renal adverse effects (RAEs) of sunitinib have not been investigated. The aim of this study was to determine the incidence and risk factors of RAEs (proteinuria [PU] and renal insufficiency [RI]) and to investigate the relationship between PU and antitumor efficacy.
Methods: We performed a retrospective review of medical records of patients who had received sunitinib for more than 3 months.
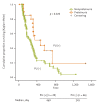
Results: One hundred and fifty-five patients (mean age, 58.7 ± 12.6 years) were enrolled, and the mean baseline creatinine level was 1.24 mg/dL. PU developed in 15 of 111 patients, and preexisting PU was aggravated in six of 111 patients. Only one patient developed typical nephrotic syndrome. Following discontinuation of sunitinib, PU was improved in 12 of 17 patients but persisted in five of 17 patients. RI occurred in 12 of 155 patients, and the maximum creatinine level was 3.31 mg/dL. RI improved in two of 12 patients but persisted in 10 of 12 patients. Risk factors for PU were hypertension, dyslipidemia, and chronic kidney disease. Older age was a risk factor for RI. The median progression-free survival was significantly better for patients who showed PU.
Conclusions: The incidence of RAEs associated with sunitinib was lower than those of previous reports. The severity of RAEs was mild to moderate, and partially reversible after cessation of sunitinib. We suggest that blood pressure, urinalysis, and renal function in patients receiving sunitinib should be monitored closely.
Keywords: Acute kidney injury; Proteinuria; Sunitinib.
Conflict of interest statement
No potential conflict of interest relevant to this article is reported.
Figures
Comment in
-
Risks associated with sunitinib use and monitoring to improve patient outcomes.Korean J Intern Med. 2014 Jan;29(1):23-6. doi: 10.3904/kjim.2014.29.1.23. Epub 2014 Jan 2. Korean J Intern Med. 2014. PMID: 24574829 Free PMC article. No abstract available.
Similar articles
-
Sunitinib and sorafenib in metastatic renal cell carcinoma patients with renal insufficiency.Ann Oncol. 2010 Aug;21(8):1618-1622. doi: 10.1093/annonc/mdp603. Epub 2010 Jan 20. Ann Oncol. 2010. PMID: 20089567
-
Efficacy and toxicity of sunitinib in patients with metastatic renal cell carcinoma with renal insufficiency.Eur J Cancer. 2014 Mar;50(4):746-52. doi: 10.1016/j.ejca.2013.11.029. Epub 2013 Dec 11. Eur J Cancer. 2014. PMID: 24332573
-
Hypertension as a biomarker of efficacy in patients with metastatic renal cell carcinoma treated with sunitinib.J Natl Cancer Inst. 2011 May 4;103(9):763-73. doi: 10.1093/jnci/djr128. Epub 2011 Apr 28. J Natl Cancer Inst. 2011. PMID: 21527770 Free PMC article.
-
Response to sorafenib after sunitinib-induced acute heart failure in a patient with metastatic renal cell carcinoma: case report and review of the literature.Pharmacotherapy. 2009 Apr;29(4):473-8. doi: 10.1592/phco.29.4.473. Pharmacotherapy. 2009. PMID: 19323623 Review.
-
Sunitinib malate for the treatment of metastatic renal cell carcinoma and gastrointestinal stromal tumors.Clin Ther. 2007 Jul;29(7):1338-53. doi: 10.1016/j.clinthera.2007.07.022. Clin Ther. 2007. PMID: 17825686 Review.
Cited by
-
Impact of diabetes mellitus and hypertension on renal function during first-line targeted therapy for metastatic renal cell carcinoma: a retrospective multicenter study.Transl Androl Urol. 2024 Sep 30;13(9):1912-1921. doi: 10.21037/tau-24-231. Epub 2024 Sep 26. Transl Androl Urol. 2024. PMID: 39434726 Free PMC article.
-
Association Between Renal Adverse Effects and Mortality in Patients With Hepatocellular Carcinoma Treated With Lenvatinib.In Vivo. 2021 May-Jun;35(3):1647-1653. doi: 10.21873/invivo.12423. In Vivo. 2021. PMID: 33910848 Free PMC article.
-
Evaluation of renal function change during first-line tyrosine kinase inhibitor therapy for metastatic renal cell carcinoma.Jpn J Clin Oncol. 2017 Dec 1;47(12):1175-1181. doi: 10.1093/jjco/hyx161. Jpn J Clin Oncol. 2017. PMID: 29140528 Free PMC article.
-
Identification of VEGF Signaling Inhibition-Induced Glomerular Injury in Rats through Site-Specific Urinary Biomarkers.Int J Mol Sci. 2021 Nov 23;22(23):12629. doi: 10.3390/ijms222312629. Int J Mol Sci. 2021. PMID: 34884436 Free PMC article.
-
Renal toxicity of targeted therapies for renal cell carcinoma in patients with normal and impaired kidney function.Cancer Chemother Pharmacol. 2021 Jun;87(6):723-742. doi: 10.1007/s00280-021-04260-y. Epub 2021 Mar 25. Cancer Chemother Pharmacol. 2021. PMID: 33768301 Free PMC article. Review.
References
-
- Boehm S, Rothermundt C, Hess D, Joerger M. Antiangiogenic drugs in oncology: a focus on drug safety and the elderly: a mini-review. Gerontology. 2010;56:303–309. - PubMed
-
- Gurevich F, Perazella MA. Renal effects of anti-angiogenesis therapy: update for the internist. Am J Med. 2009;122:322–328. - PubMed
-
- Oudard S, Beuselinck B, Decoene J, Albers P. Sunitinib for the treatment of metastatic renal cell carcinoma. Cancer Treat Rev. 2011;37:178–184. - PubMed
-
- Rini BI, Garcia JA, Cooney MM, et al. Toxicity of sunitinib plus bevacizumab in renal cell carcinoma. J Clin Oncol. 2010;28:e284–e285. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
