Targeted therapies in development for non-small cell lung cancer
- PMID: 24574860
- PMCID: PMC3927069
- DOI: 10.4103/1477-3163.123972
Targeted therapies in development for non-small cell lung cancer
Abstract
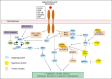
The iterative discovery in various malignancies during the past decades that a number of aberrant tumorigenic processes and signal transduction pathways are mediated by "druggable" protein kinases has led to a revolutionary change in drug development. In non-small cell lung cancer (NSCLC), the ErbB family of receptors (e.g., EGFR [epidermal growth factor receptor], HER2 [human epidermal growth factor receptor 2]), RAS (rat sarcoma gene), BRAF (v-raf murine sarcoma viral oncogene homolog B1), MAPK (mitogen-activated protein kinase) c-MET (c-mesenchymal-epithelial transition), FGFR (fibroblast growth factor receptor), DDR2 (discoidin domain receptor 2), PIK3CA (phosphatidylinositol-4,5-bisphosphate3-kinase, catalytic subunit alpha)), PTEN (phosphatase and tensin homolog), AKT (protein kinase B), ALK (anaplastic lym phoma kinase), RET (rearranged during transfection), ROS1 (reactive oxygen species 1) and EPH (erythropoietin-producing hepatoma) are key targets of various agents currently in clinical development. These oncogenic targets exert their selective growth advantage through various intercommunicating pathways, such as through RAS/RAF/MEK, phosphoinositide 3-kinase/AKT/mammalian target of rapamycin and SRC-signal transduction and transcription signaling. The recent clinical studies, EGFR tyrosine kinase inhibitors and crizotinib were considered as strongly effective targeted therapies in metastatic NSCLC. Currently, five molecular targeted agents were approved for treatment of advanced NSCLC: Gefitinib, erlotinib and afatinib for positive EGFR mutation, crizotinib for positive echinoderm microtubule-associated protein-like 4 (EML4)-ALK translocation and bevacizumab. Moreover, oncogenic mutant proteins are subject to regulation by protein trafficking pathways, specifically through the heat shock protein 90 system. Drug combinations affecting various nodes in these signaling and intracellular processes are predicted and demonstrated to be synergistic and advantageous in overcoming treatment resistance compared with monotherapy approaches. Understanding the role of the tumor microenvironment in the development and maintenance of the malignant phenotype provided additional therapeutic approaches as well. More recently, improved knowledge on tumor immunology has set the stage for promising immunotherapies in NSCLC. This review will focus on the rationale for the development of targeted therapies in NSCLC and the various strategies employed in preventing or overcoming the inevitable occurrence of treatment resistance.
Keywords: Drug resistance; heat shock protein 90 inhibitors; non-small cell lung cancer; programmed cell death-1 receptor inhibitors; protein kinase inhibitors.
Conflict of interest statement
Figures
Similar articles
-
Mechanisms of resistance to EGFR tyrosine kinase inhibitors.Acta Pharm Sin B. 2015 Sep;5(5):390-401. doi: 10.1016/j.apsb.2015.07.001. Epub 2015 Jul 26. Acta Pharm Sin B. 2015. PMID: 26579470 Free PMC article. Review.
-
Management of advanced non-small cell lung cancers with known mutations or rearrangements: latest evidence and treatment approaches.Ther Adv Respir Dis. 2016 Apr;10(2):113-29. doi: 10.1177/1753465815617871. Epub 2015 Nov 30. Ther Adv Respir Dis. 2016. PMID: 26620497 Free PMC article. Review.
-
Concomitant occurrence of EGFR (epidermal growth factor receptor) and KRAS (V-Ki-ras2 Kirsten rat sarcoma viral oncogene homolog) mutations in an ALK (anaplastic lymphoma kinase)-positive lung adenocarcinoma patient with acquired resistance to crizotinib: a case report.BMC Res Notes. 2013 Nov 26;6:489. doi: 10.1186/1756-0500-6-489. BMC Res Notes. 2013. PMID: 24279718 Free PMC article.
-
The ErbB/HER family of protein-tyrosine kinases and cancer.Pharmacol Res. 2014 Jan;79:34-74. doi: 10.1016/j.phrs.2013.11.002. Epub 2013 Nov 20. Pharmacol Res. 2014. PMID: 24269963 Review.
-
Coexistence of PIK3CA and other oncogene mutations in lung adenocarcinoma-rationale for comprehensive mutation profiling.Mol Cancer Ther. 2012 Feb;11(2):485-91. doi: 10.1158/1535-7163.MCT-11-0692. Epub 2011 Dec 1. Mol Cancer Ther. 2012. PMID: 22135231 Free PMC article.
Cited by
-
Afatinib demonstrates remarkable activity against HER2-amplified uterine serous endometrial cancer in vitro and in vivo.Br J Cancer. 2014 Oct 28;111(9):1750-6. doi: 10.1038/bjc.2014.519. Epub 2014 Sep 30. Br J Cancer. 2014. PMID: 25268372 Free PMC article.
-
Activation of PPARγ in Myeloid Cells Promotes Progression of Epithelial Lung Tumors through TGFβ1.Mol Cancer Res. 2019 Aug;17(8):1748-1758. doi: 10.1158/1541-7786.MCR-19-0236. Epub 2019 May 14. Mol Cancer Res. 2019. PMID: 31088909 Free PMC article.
-
Anti-proliferative effects of evodiamine in human lung cancer cells.J Cancer Prev. 2014 Mar;19(1):7-13. doi: 10.15430/jcp.2014.19.1.7. J Cancer Prev. 2014. PMID: 25337567 Free PMC article.
-
Targeted therapies for the treatment of non-small-cell lung cancer: Monoclonal antibodies and biological inhibitors.Hum Vaccin Immunother. 2017 Apr 3;13(4):843-853. doi: 10.1080/21645515.2016.1249551. Epub 2016 Nov 10. Hum Vaccin Immunother. 2017. PMID: 27831000 Free PMC article. Review.
-
Targeting CD70 in combination with chemotherapy to enhance the anti-tumor immune effects in non-small cell lung cancer.Oncoimmunology. 2023 Mar 23;12(1):2192100. doi: 10.1080/2162402X.2023.2192100. eCollection 2023. Oncoimmunology. 2023. PMID: 36970072 Free PMC article.
References
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Mountain CF. The international system for staging lung cancer. Semin Surg Oncol. 2000;18:106–15. - PubMed
-
- Burgess DJ. Cancer genetics: Initially complex, always heterogeneous. Nat Rev Cancer. 2011;11:153. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

