Chronic cerebral hypoperfusion causes decrease of O-GlcNAcylation, hyperphosphorylation of tau and behavioral deficits in mice
- PMID: 24575038
- PMCID: PMC3918671
- DOI: 10.3389/fnagi.2014.00010
Chronic cerebral hypoperfusion causes decrease of O-GlcNAcylation, hyperphosphorylation of tau and behavioral deficits in mice
Abstract
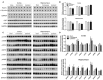
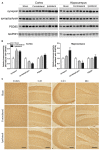
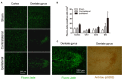
Chronic cerebral hypoperfusion (CCH) is one of the causes of vascular dementia (VaD) and is also an etiological factor for Alzheimer's disease (AD). However, how CCH causes cognitive impairment and contributes to Alzheimer's pathology is poorly understood. Here we produced a mouse model of CCH by unilateral common carotid artery occlusion (UCCAO) and studied the behavioral changes and brain abnormalities in mice 2.5 months after UCCAO. We found that CCH caused significant short-term memory deficits and mild long-term spatial memory impairment, as well as decreased level of protein O-GlcNAcylation, increased level of tau phosphorylation, dysregulated synaptic proteins and insulin signaling, and selective neurodegeneration in the brain. These findings provide mechanistic insight into the effects of CCH on memory and cognition and the likely link between AD and VaD.
Keywords: Alzheimer’s disease; O-GlcNAcylation; brain insulin signaling; chronic cerebral hypoperfusion; cognitive impairment; neurodegeneration; synaptic plasticity markers; tau phosphorylation.
Figures




Similar articles
-
Thiamme2-G, a Novel O-GlcNAcase Inhibitor, Reduces Tau Hyperphosphorylation and Rescues Cognitive Impairment in Mice.J Alzheimers Dis. 2021;81(1):273-286. doi: 10.3233/JAD-201450. J Alzheimers Dis. 2021. PMID: 33814439
-
The Involvement of Canonical Wnt Signaling in Memory Impairment Induced by Chronic Cerebral Hypoperfusion in Mice.Transl Stroke Res. 2020 Aug;11(4):734-746. doi: 10.1007/s12975-019-00748-1. Epub 2020 Jan 20. Transl Stroke Res. 2020. PMID: 31960287
-
Enriched environment prevents cognitive impairment and tau hyperphosphorylation after chronic cerebral hypoperfusion.Curr Neurovasc Res. 2012 Aug;9(3):176-84. doi: 10.2174/156720212801618974. Curr Neurovasc Res. 2012. PMID: 22621232
-
From chronic cerebral hypoperfusion to Alzheimer-like brain pathology and neurodegeneration.Cell Mol Neurobiol. 2015 Jan;35(1):101-10. doi: 10.1007/s10571-014-0127-9. Epub 2014 Oct 29. Cell Mol Neurobiol. 2015. PMID: 25352419 Free PMC article. Review.
-
Cerebral hypoperfusion and glucose hypometabolism: Key pathophysiological modulators promote neurodegeneration, cognitive impairment, and Alzheimer's disease.J Neurosci Res. 2017 Apr;95(4):943-972. doi: 10.1002/jnr.23777. Epub 2016 Jun 27. J Neurosci Res. 2017. PMID: 27350397 Review.
Cited by
-
Cerebral Small Vessel Disease and Alzheimer's Disease: A Review.Front Neurol. 2020 Aug 25;11:927. doi: 10.3389/fneur.2020.00927. eCollection 2020. Front Neurol. 2020. PMID: 32982937 Free PMC article. Review.
-
Potential mechanisms and implications for the formation of tau oligomeric strains.Crit Rev Biochem Mol Biol. 2016 Nov/Dec;51(6):482-496. doi: 10.1080/10409238.2016.1226251. Epub 2016 Sep 21. Crit Rev Biochem Mol Biol. 2016. PMID: 27650389 Free PMC article. Review.
-
The Potential Role of MicroRNA-124 in Cerebral Ischemia Injury.Int J Mol Sci. 2019 Dec 23;21(1):120. doi: 10.3390/ijms21010120. Int J Mol Sci. 2019. PMID: 31878035 Free PMC article. Review.
-
Impairments in Brain Bioenergetics in Aging and Tau Pathology: A Chicken and Egg Situation?Cells. 2021 Sep 24;10(10):2531. doi: 10.3390/cells10102531. Cells. 2021. PMID: 34685510 Free PMC article. Review.
-
Mumefural Improves Recognition Memory and Alters ERK-CREB-BDNF Signaling in a Mouse Model of Chronic Cerebral Hypoperfusion.Nutrients. 2023 Jul 24;15(14):3271. doi: 10.3390/nu15143271. Nutrients. 2023. PMID: 37513692 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

