Connexin hemichannels in the lens
- PMID: 24575044
- PMCID: PMC3920103
- DOI: 10.3389/fphys.2014.00020
Connexin hemichannels in the lens
Abstract
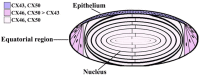
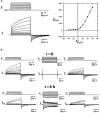
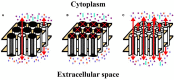
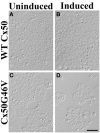
The normal function and survival of cells in the avascular lens is facilitated by intercellular communication through an extensive network of gap junctions formed predominantly by three connexins (Cx43, Cx46, and Cx50). In expression systems, these connexins can all induce hemichannel currents, but other lens proteins (e.g., pannexin1) can also induce similar currents. Hemichannel currents have been detected in isolated lens fiber cells. These hemichannels may make significant contributions to normal lens physiology and pathophysiology. Studies of some connexin mutants linked to congenital cataracts have implicated hemichannels with aberrant voltage-dependent gating or modulation by divalent cations in disease pathogenesis. Hemichannels may also contribute to age- and disease-related cataracts.
Keywords: cataract; connexin46; connexin50; gap junction; lens.
Figures
References
-
- Arora A., Minogue P. J., Liu X., Reddy M. A., Ainsworth J. R., Bhattacharya S. S., et al. (2006). A novel GJA8 mutation is associated with autosomal dominant lamellar pulverulent cataract: further evidence for gap junction dysfunction in human cataract. J. Med. Genet. 43, e2 10.1136/jmg.2005.034108 - DOI - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

