Stressful presentations: mild cold stress in laboratory mice influences phenotype of dendritic cells in naïve and tumor-bearing mice
- PMID: 24575090
- PMCID: PMC3918933
- DOI: 10.3389/fimmu.2014.00023
Stressful presentations: mild cold stress in laboratory mice influences phenotype of dendritic cells in naïve and tumor-bearing mice
Abstract
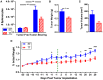
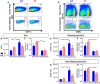
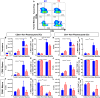
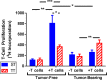
The ability of dendritic cells (DCs) to stimulate and regulate T cells is critical to effective anti-tumor immunity. Therefore, it is important to fully recognize any inherent factors which may influence DC function under experimental conditions, especially in laboratory mice since they are used so heavily to model immune responses. The goals of this report are to 1) briefly summarize previous work revealing how DCs respond to various forms of physiological stress and 2) to present new data highlighting the potential for chronic mild cold stress inherent to mice housed at the required standard ambient temperatures to influence baseline DCs properties in naïve and tumor-bearing mice. As recent data from our group shows that CD8(+) T cell function is significantly altered by chronic mild cold stress and since DC function is crucial for CD8(+) T cell activation, we wondered whether housing temperature may also be influencing DC function. Here we report that there are several significant phenotypical and functional differences among DC subsets in naïve and tumor-bearing mice housed at either standard housing temperature or at a thermoneutral ambient temperature, which significantly reduces the extent of cold stress. The new data presented here strongly suggests that, by itself, the housing temperature of mice can affect fundamental properties and functions of DCs. Therefore differences in basal levels of stress due to housing should be taken into consideration when interpreting experiments designed to evaluate the impact of additional variables, including other stressors on DC function.
Keywords: anti-tumor immunity; cold stress; mouse models of cancer; norepinephrine; thermoregulation.
Figures

Similar articles
-
Baseline tumor growth and immune control in laboratory mice are significantly influenced by subthermoneutral housing temperature.Proc Natl Acad Sci U S A. 2013 Dec 10;110(50):20176-81. doi: 10.1073/pnas.1304291110. Epub 2013 Nov 18. Proc Natl Acad Sci U S A. 2013. PMID: 24248371 Free PMC article.
-
The Impact of Housing Temperature-Induced Chronic Stress on Preclinical Mouse Tumor Models and Therapeutic Responses: An Important Role for the Nervous System.Adv Exp Med Biol. 2017;1036:173-189. doi: 10.1007/978-3-319-67577-0_12. Adv Exp Med Biol. 2017. PMID: 29275472 Free PMC article.
-
How murine models of human disease and immunity are influenced by housing temperature and mild thermal stress.Temperature (Austin). 2022 Jul 15;10(2):166-178. doi: 10.1080/23328940.2022.2093561. eCollection 2023. Temperature (Austin). 2022. PMID: 37332306 Free PMC article. Review.
-
Housing temperature influences the pattern of heat shock protein induction in mice following mild whole body hyperthermia.Int J Hyperthermia. 2014 Dec;30(8):540-6. doi: 10.3109/02656736.2014.981300. Int J Hyperthermia. 2014. PMID: 25430986 Free PMC article.
-
Mild cold-stress depresses immune responses: Implications for cancer models involving laboratory mice.Bioessays. 2014 Sep;36(9):884-91. doi: 10.1002/bies.201400066. Epub 2014 Jul 25. Bioessays. 2014. PMID: 25066924 Free PMC article. Review.
Cited by
-
Targeting beta-adrenergic receptor pathways in melanoma: how stress modulates oncogenic immunity.Melanoma Res. 2024 Apr 1;34(2):89-95. doi: 10.1097/CMR.0000000000000943. Epub 2023 Dec 4. Melanoma Res. 2024. PMID: 38051781 Free PMC article. Review.
-
Neuroscience of cancer: unraveling the complex interplay between the nervous system, the tumor and the tumor immune microenvironment.Mol Cancer. 2025 Jan 17;24(1):24. doi: 10.1186/s12943-024-02219-0. Mol Cancer. 2025. PMID: 39825376 Free PMC article. Review.
-
Beta-Adrenergic Blockade in Advanced Non-Small Cell Lung Cancer Patients Receiving Immunotherapy: A Multicentric Study.Cureus. 2024 Jan 13;16(1):e52194. doi: 10.7759/cureus.52194. eCollection 2024 Jan. Cureus. 2024. PMID: 38348009 Free PMC article.
-
Interactions Between Housing Density and Ambient Temperature in the Cage Environment: Effects on Mouse Physiology and Behavior.J Am Assoc Lab Anim Sci. 2015 Nov;54(6):708-17. J Am Assoc Lab Anim Sci. 2015. PMID: 26632780 Free PMC article.
-
Standard sub-thermoneutral caging temperature influences radiosensitivity of hematopoietic stem and progenitor cells.PLoS One. 2015 Mar 20;10(3):e0120078. doi: 10.1371/journal.pone.0120078. eCollection 2015. PLoS One. 2015. PMID: 25793392 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

