Natural killer cells and neuroblastoma: tumor recognition, escape mechanisms, and possible novel immunotherapeutic approaches
- PMID: 24575100
- PMCID: PMC3921882
- DOI: 10.3389/fimmu.2014.00056
Natural killer cells and neuroblastoma: tumor recognition, escape mechanisms, and possible novel immunotherapeutic approaches
Abstract
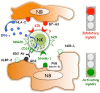
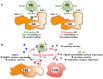
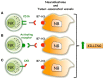
Neuroblastoma (NB) is the most common extra-cranial solid tumor of childhood and arises from developing sympathetic nervous system. Most primary tumors localize in the abdomen, the adrenal gland, or lumbar sympathetic ganglia. Amplification in tumor cells of MYCN, the major oncogenic driver, patients' age over 18 months, and the presence at diagnosis of a metastatic disease (stage IV, M) identify NB at high risk of treatment failure. Conventional therapies did not significantly improve the overall survival of these patients. Moreover, the limited landscape of somatic mutations detected in NB is hampering the development of novel pharmacological approaches. Major efforts aim to identify novel NB-associated surface molecules that activate immune responses and/or direct drugs to tumor cells and tumor-associated vessels. PVR (Poliovirus Receptor) and B7-H3 are promising targets, since they are expressed by most high-risk NB, are upregulated in tumor vasculature and are essential for tumor survival/invasiveness. PVR is a ligand of DNAM-1 activating receptor that triggers the cytolytic activity of natural killer (NK) cells against NB. In animal models, targeting of PVR with an attenuated oncolytic poliovirus induced tumor regression and elimination. Also B7-H3 was successfully targeted in preclinical studies and is now being tested in phase I/II clinical trials. B7-H3 down-regulates NK cytotoxicity, providing NB with a mechanism of escape from immune response. The immunosuppressive potential of NB can be enhanced by the release of soluble factors that impair NK cell function and/or recruitment. Among these, TGF-β1 modulates the cytotoxicity receptors and the chemokine receptor repertoire of NK cells. Here, we summarize the current knowledge on the main cell surface molecules and soluble mediators that modulate the function of NK cells in NB, considering the pros and cons that must be taken into account in the design of novel NK cell-based immunotherapeutic approaches.
Keywords: B7-H3; PVR; TGF-beta; chemokine receptors; immunotherapeutic approaches; natural killer cells; neuroblastoma; tumor escape mechanisms.
Figures
Similar articles
-
Identification of neuroblastoma cell lines with uncommon TAZ+/mesenchymal stromal cell phenotype with strong suppressive activity on natural killer cells.J Immunother Cancer. 2021 Jan;9(1):e001313. doi: 10.1136/jitc-2020-001313. J Immunother Cancer. 2021. PMID: 33452207 Free PMC article.
-
MYCN is an immunosuppressive oncogene dampening the expression of ligands for NK-cell-activating receptors in human high-risk neuroblastoma.Oncoimmunology. 2017 Apr 20;6(6):e1316439. doi: 10.1080/2162402X.2017.1316439. eCollection 2017. Oncoimmunology. 2017. PMID: 28680748 Free PMC article.
-
Neuroblastoma-derived TGF-β1 modulates the chemokine receptor repertoire of human resting NK cells.J Immunol. 2013 May 15;190(10):5321-8. doi: 10.4049/jimmunol.1202693. Epub 2013 Apr 10. J Immunol. 2013. PMID: 23576682
-
Natural killer cells in neuroblastoma: immunological insights and therapeutic perspectives.Cancer Metastasis Rev. 2024 Dec;43(4):1401-1417. doi: 10.1007/s10555-024-10212-8. Epub 2024 Sep 18. Cancer Metastasis Rev. 2024. PMID: 39294470 Free PMC article. Review.
-
Immune checkpoint molecules in neuroblastoma: A clinical perspective.Semin Cancer Biol. 2022 Nov;86(Pt 2):247-258. doi: 10.1016/j.semcancer.2022.06.013. Epub 2022 Jul 3. Semin Cancer Biol. 2022. PMID: 35787940 Review.
Cited by
-
NK Cells, Tumor Cell Transition, and Tumor Progression in Solid Malignancies: New Hints for NK-Based Immunotherapy?J Immunol Res. 2016;2016:4684268. doi: 10.1155/2016/4684268. Epub 2016 May 12. J Immunol Res. 2016. PMID: 27294158 Free PMC article. Review.
-
Hypoxia Predicts Poor Prognosis in Neuroblastoma Patients and Associates with Biological Mechanisms Involved in Telomerase Activation and Tumor Microenvironment Reprogramming.Cancers (Basel). 2020 Aug 19;12(9):2343. doi: 10.3390/cancers12092343. Cancers (Basel). 2020. PMID: 32825087 Free PMC article.
-
More than the genes, the tumor microenvironment in neuroblastoma.Cancer Lett. 2016 Sep 28;380(1):304-14. doi: 10.1016/j.canlet.2015.11.017. Epub 2015 Nov 17. Cancer Lett. 2016. PMID: 26597947 Free PMC article. Review.
-
Biological Insight and Recent Advancement in the Treatment of Neuroblastoma.Int J Mol Sci. 2023 May 9;24(10):8470. doi: 10.3390/ijms24108470. Int J Mol Sci. 2023. PMID: 37239815 Free PMC article. Review.
-
The Thermal Dose of Photothermal Therapy Generates Differential Immunogenicity in Human Neuroblastoma Cells.Cancers (Basel). 2022 Mar 11;14(6):1447. doi: 10.3390/cancers14061447. Cancers (Basel). 2022. PMID: 35326601 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

