Pharmacokinetics of PEGylated Recombinant Human Erythropoietin in Rats
- PMID: 24575314
- PMCID: PMC3913096
- DOI: 10.1155/2014/918686
Pharmacokinetics of PEGylated Recombinant Human Erythropoietin in Rats
Abstract
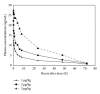
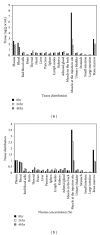
rHuEPO plays a central role as chemicals for the treatment of many diseases. Due to its short half-life, the main aim for this pharmacokinetic study is to investigate a newly developed PEG-rHuEPO with large molecular weight in SD rats. After a single intramuscular administration of different doses of 125I-PEG-rHuEPO, pharmacokinetic parameters, tissue distribution, and excretion were analyzed. In in vivo half-life time measured after 125I-PEG-rHuEPO administration at the doses of 1, 2, and 3 μg/kg, t1/2α was 1.90, 1.19, and 2.50 hours, respectively, whereas t1/2β was 22.37, 26.21, and 20.92 hours, respectively; at 8, 24, and 48 hours after intramuscular administration, PEG-rHuEPO was distributed to all of the examined tissues, however, with high concentrations of radioactivity, only in plasma, blood, muscle at the administration site, and bone marrow. Following a 2 μg/kg single intramuscular administration, approximately 21% of the radiolabeled dose was recovered after almost seven days of study. Urine was the major route of excretion; 20% of the administered dose was recovered in the urine, while excretion in the feces was less than 1.4%. Therefore, this PEG-rHuEPO has potential to be clinically used and could reduce frequency of injection.
Figures

Similar articles
-
Plasma levels, tissue distribution, and excretion of radioactivity after single-dose administration of ((3)H)-oleic acid added to D-004, a lipid extract of the fruit of Roystonea regia, in rats.Curr Ther Res Clin Exp. 2006 Nov;67(6):406-19. doi: 10.1016/j.curtheres.2006.12.005. Curr Ther Res Clin Exp. 2006. PMID: 24678113 Free PMC article.
-
Pharmacokinetic study of darbepoetin alfa: absorption, distribution, and excretion after a single intravenous and subcutaneous administration to rats.Xenobiotica. 2007 Jan;37(1):74-90. doi: 10.1080/00498250600987929. Xenobiotica. 2007. PMID: 17178635
-
Pharmacokinetics of PEGylated recombinant human endostatin (M2ES) in rats.Acta Pharmacol Sin. 2015 Jul;36(7):847-54. doi: 10.1038/aps.2015.16. Epub 2015 Jun 1. Acta Pharmacol Sin. 2015. PMID: 26027657 Free PMC article.
-
Absorption, distribution, metabolism and excretion of glucosamine sulfate. A review.Arzneimittelforschung. 2001 Sep;51(9):699-725. doi: 10.1055/s-0031-1300105. Arzneimittelforschung. 2001. PMID: 11642003 Review.
-
Novel erythropoiesis stimulating protein for managing the anemia of chronic kidney disease.Am J Kidney Dis. 2001 Dec;38(6):1390-7. doi: 10.1053/ajkd.2001.29264. Am J Kidney Dis. 2001. PMID: 11728981 Review.
Cited by
-
A comparative pharmacokinetic and pharmacodynamic study of two novel Cuban PEGylated rHuEPO versus MIRCERA® and ior®EPOCIM.J Pharm Pharmacogn Res. 2018 May-Jun;6(3):179-190. Epub 2018 Feb 23. J Pharm Pharmacogn Res. 2018. PMID: 30739984 Free PMC article.
-
Advances in Understanding the Effects of Erythropoietin on Renal Fibrosis.Front Med (Lausanne). 2020 Feb 21;7:47. doi: 10.3389/fmed.2020.00047. eCollection 2020. Front Med (Lausanne). 2020. PMID: 32154256 Free PMC article. Review.
References
-
- Pfeilschifter J, Huwiler A. Erythropoietin is more than just a promoter of erythropoiesis. Journal of the American Society of Nephrology. 2004;15(8):2240–2241. - PubMed
-
- Connie MK, Dorie M, Debora K, et al. Pharmacokinetics and pharmacodynamics of an EPO-mimetic fusion protein in a model of chronic renal insufficiency anemia. The Open Hematology Journal. 2010;4:17–20.
-
- Greer JP, Foerster J, Lukens JN, et al. Wintrobe's Clinical Hematology. Baltimore, Md, USA: Lippincott Williams & Wilkins; 2003.
-
- Fisher JW. Erythropoietin: physiology and pharmacology update. Experimental Biology and Medicine. 2003;228(1):1–14. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

