Bioartificial Renal Epithelial Cell System (BRECS): A Compact, Cryopreservable Extracorporeal Renal Replacement Device
- PMID: 24575327
- PMCID: PMC3933030
- DOI: 10.3727/215517912X653328
Bioartificial Renal Epithelial Cell System (BRECS): A Compact, Cryopreservable Extracorporeal Renal Replacement Device
Abstract
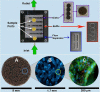
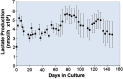
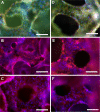
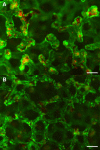
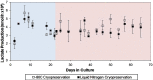
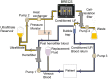
Renal cell therapy has shown clinical efficacy in the treatment of acute renal failure (ARF) and promise for treatment of end-stage renal disease (ESRD) by supplementing conventional small solute clearance (hemodialysis or hemofiltration) with endocrine and metabolic function provided by cells maintained in an extracorporeal circuit. A major obstacle in the widespread adoption of this therapeutic approach is the lack of a cryopreservable system to enable distribution, storage, and therapeutic use at point of care facilities. This report details the design, fabrication, and assessment of a Bioartificial Renal Epithelial Cell System (BRECS), the first all-in-one culture vessel, cryostorage device, and cell therapy delivery system. The BRECS was loaded with up to 20 cell-seeded porous disks, which were maintained by perfusion culture. Once cells reached over 5 × 106 cells/disk for a total therapeutic dose of approximately 108 cells, the BRECS was cryopreserved for storage at -80°C or -140°C. The BRECS was rapidly thawed, and perfusion culture was resumed. Near precryopreservation values of cell viability, metabolic activity, and differentiated phenotype of functional renal cells were confirmed post-reconstitution. This technology could be extended to administer other cell-based therapies where metabolic, regulatory, or secretion functions can be leveraged in an immunoisolated extracorporeal circuit.
Keywords: Acute renal failure; Bioreactor; Cryopreservation; End-stage renal disease; Extracorporeal cell therapy; Progenitor.
Figures


Similar articles
-
Development of a wearable bioartificial kidney using the Bioartificial Renal Epithelial Cell System (BRECS).J Tissue Eng Regen Med. 2017 Nov;11(11):3048-3055. doi: 10.1002/term.2206. Epub 2016 Nov 18. J Tissue Eng Regen Med. 2017. PMID: 27860413
-
Bioengineered Renal Cell Therapy Device for Clinical Translation.ASAIO J. 2017 May/Jun;63(3):305-315. doi: 10.1097/MAT.0000000000000485. ASAIO J. 2017. PMID: 27922886 Free PMC article.
-
A bio-artificial renal epithelial cell system conveys survival advantage in a porcine model of septic shock.J Tissue Eng Regen Med. 2017 Mar;11(3):649-657. doi: 10.1002/term.1961. Epub 2014 Nov 25. J Tissue Eng Regen Med. 2017. PMID: 25424193 Free PMC article.
-
Bioartificial kidney for full renal replacement therapy.Semin Nephrol. 2000 Jan;20(1):71-82. Semin Nephrol. 2000. PMID: 10651220 Review.
-
The bioartificial kidney in the treatment of acute renal failure.Kidney Int Suppl. 2002 May;(80):121-5. doi: 10.1046/j.1523-1755.61.s80.22.x. Kidney Int Suppl. 2002. PMID: 11982826 Review.
Cited by
-
Current strategies and challenges in engineering a bioartificial kidney.Front Biosci (Elite Ed). 2015 Jan 1;7(2):215-28. doi: 10.2741/E729. Front Biosci (Elite Ed). 2015. PMID: 25553375 Free PMC article. Review.
-
Portable, wearable and implantable artificial kidney systems: needs, opportunities and challenges.Nat Rev Nephrol. 2023 Aug;19(8):481-490. doi: 10.1038/s41581-023-00726-9. Epub 2023 Jun 5. Nat Rev Nephrol. 2023. PMID: 37277461 Free PMC article. Review.
-
Current Status and Future of Artificial Kidney in Humans.Indian J Nephrol. 2022 Nov-Dec;32(6):531-538. doi: 10.4103/ijn.ijn_240_21. Epub 2022 Dec 1. Indian J Nephrol. 2022. PMID: 36704585 Free PMC article. Review.
-
Bioengineering in renal transplantation: technological advances and novel options.Pediatr Nephrol. 2018 Jul;33(7):1105-1111. doi: 10.1007/s00467-017-3706-4. Epub 2017 Jun 6. Pediatr Nephrol. 2018. PMID: 28589209 Review.
-
The bioartificial kidney: current status and future promise.Pediatr Nephrol. 2014 Mar;29(3):343-51. doi: 10.1007/s00467-013-2467-y. Epub 2013 Apr 26. Pediatr Nephrol. 2014. PMID: 23619508 Review.
References
-
- Atala A. Tissue engineering and regenerative medicine: Concepts for clinical application. Rejuvenation Res. 7(1):15–31; 2004. - PubMed
-
- Brodie J. C.; Humes H. D. Stem cell approaches for the treatment of renal failure. Pharmacol. Rev. 57(3):299–313; 2005. - PubMed
-
- Casino F. G. [Dialysis dose quantification in critically ill patients]. G. Ital. Nefrol. 27(4):383–390; 2010. - PubMed
-
- Davenport A.; Gura V.; Ronco C.; Beizai M.; Ezon C.; Rambod E. A wearable haemodialysis device for patients with end-stage renal failure: A pilot study. Lancet 370(9604):2005–2010; 2007. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

