Obstetric Pharmacokinetic Dosing Studies are Urgently Needed
- PMID: 24575394
- PMCID: PMC3920104
- DOI: 10.3389/fped.2014.00009
Obstetric Pharmacokinetic Dosing Studies are Urgently Needed
Abstract
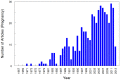
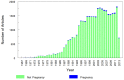
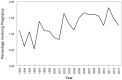
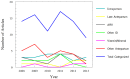
Use of pharmacotherapy during pregnancy is common and increasing. Physiologic changes during pregnancy may significantly alter the overall systemic drug exposure, necessitating dose changes. A search of PubMed for pharmacokinetic clinical trials showed 494 publications during pregnancy out of 35,921 total pharmacokinetic published studies (1.29%), from the late 1960s through August 31, 2013. Closer examination of pharmacokinetic studies in pregnant women published since 2008 (81 studies) revealed that about a third of the trials were for treatment of acute labor and delivery issues, a third included studies of infectious disease treatment during pregnancy, and the remaining third were for varied ante-partum indications. Approximately, two-thirds of these recent studies were primarily funded by government agencies worldwide, one-quarter were supported by private non-profit foundations or combinations of government and private funding, and slightly <10% were supported by pharmaceutical industry. As highlighted in this review, vast gaps exist in pharmacology information and evidence for appropriate dosing of medications in pregnant women. This lack of knowledge and understanding of drug disposition throughout pregnancy place both the mother and the fetus at risk for avoidable therapeutic misadventures - suboptimal efficacy or excess toxicity - with medication use in pregnancy. Increased efforts to perform and support obstetric dosing and pharmacokinetic studies are greatly needed.
Keywords: maternal–fetal; obstetrics; pharmacokinetics; pharmacology; pregnancy.
Figures

References
-
- Ventura SJ, Curtin SC, Abma JC, Henshaw SK. Estimated pregnancy rates and rates of pregnancy outcomes for the United States, 1990-2008. Natl Vital Stat Rep (2012) 60(7):1–21 - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

