The taccalonolides and paclitaxel cause distinct effects on microtubule dynamics and aster formation
- PMID: 24576146
- PMCID: PMC4015978
- DOI: 10.1186/1476-4598-13-41
The taccalonolides and paclitaxel cause distinct effects on microtubule dynamics and aster formation
Abstract
Background: Microtubule stabilizers suppress microtubule dynamics and, at the lowest antiproliferative concentrations, disrupt the function of mitotic spindles, leading to mitotic arrest and apoptosis. At slightly higher concentrations, these agents cause the formation of multiple mitotic asters with distinct morphologies elicited by different microtubule stabilizers.
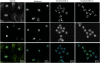
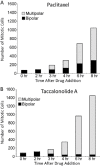
Results: We tested the hypothesis that two classes of microtubule stabilizing drugs, the taxanes and the taccalonolides, cause the formation of distinct aster structures due, in part, to differential effects on microtubule dynamics. Paclitaxel and the taccalonolides suppressed the dynamics of microtubules formed from purified tubulin as well as in live cells. Both agents suppressed microtubule dynamic instability, with the taccalonolides having a more pronounced inhibition of microtubule catastrophe, suggesting that they stabilize the plus ends of microtubules more effectively than paclitaxel. Live cell microscopy was also used to evaluate the formation and resolution of asters after drug treatment. While each drug had similar effects on initial formation, substantial differences were observed in aster resolution. Paclitaxel-induced asters often coalesced over time resulting in fewer, larger asters whereas numerous compact asters persisted once they were formed in the presence of the taccalonolides.
Conclusions: We conclude that the increased resistance of microtubule plus ends to catastrophe may play a role in the observed inability of taccalonolide-induced asters to coalesce during mitosis, giving rise to the distinct morphologies observed after exposure to these agents.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

