Mechanism of quinolone action and resistance
- PMID: 24576155
- PMCID: PMC3985860
- DOI: 10.1021/bi5000564
Mechanism of quinolone action and resistance
Abstract
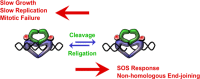
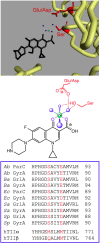
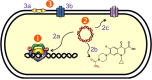
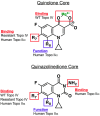
Quinolones are one of the most commonly prescribed classes of antibacterials in the world and are used to treat a variety of bacterial infections in humans. Because of the wide use (and overuse) of these drugs, the number of quinolone-resistant bacterial strains has been growing steadily since the 1990s. As is the case with other antibacterial agents, the rise in quinolone resistance threatens the clinical utility of this important drug class. Quinolones act by converting their targets, gyrase and topoisomerase IV, into toxic enzymes that fragment the bacterial chromosome. This review describes the development of the quinolones as antibacterials, the structure and function of gyrase and topoisomerase IV, and the mechanistic basis for quinolone action against their enzyme targets. It will then discuss the following three mechanisms that decrease the sensitivity of bacterial cells to quinolones. Target-mediated resistance is the most common and clinically significant form of resistance. It is caused by specific mutations in gyrase and topoisomerase IV that weaken interactions between quinolones and these enzymes. Plasmid-mediated resistance results from extrachromosomal elements that encode proteins that disrupt quinolone-enzyme interactions, alter drug metabolism, or increase quinolone efflux. Chromosome-mediated resistance results from the underexpression of porins or the overexpression of cellular efflux pumps, both of which decrease cellular concentrations of quinolones. Finally, this review will discuss recent advancements in our understanding of how quinolones interact with gyrase and topoisomerase IV and how mutations in these enzymes cause resistance. These last findings suggest approaches to designing new drugs that display improved activity against resistant strains.
Figures



References
-
- Emmerson A. M.; Jones A. M. (2003) The quinolones: Decades of development and use. J. Antimicrob. Chemother. 51(Suppl. 1), 13–20. - PubMed
-
- Mitscher L. A. (2005) Bacterial topoisomerase inhibitors: Quinolone and pyridone antibacterial agents. Chem. Rev. 105, 559–592. - PubMed
-
- Linder J. A.; Huang E. S.; Steinman M. A.; Gonzales R.; Stafford R. S. (2005) Fluoroquinolone prescribing in the United States: 1995 to 2002. Am. J. Med. 118, 259–268. - PubMed
-
- Andriole V. T. (2005) The quinolones: Past, present, and future. Clin. Infect. Dis. 41(Suppl. 2), S113–S119. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

