Establishing the pre-placodal region and breaking it into placodes with distinct identities
- PMID: 24576539
- PMCID: PMC3985045
- DOI: 10.1016/j.ydbio.2014.02.011
Establishing the pre-placodal region and breaking it into placodes with distinct identities
Abstract
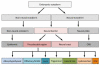
Specialized sensory organs in the vertebrate head originate from thickenings in the embryonic ectoderm called cranial sensory placodes. These placodes, as well as the neural crest, arise from a zone of ectoderm that borders the neural plate. This zone separates into a precursor field for the neural crest that lies adjacent to the neural plate, and a precursor field for the placodes, called the pre-placodal region (PPR), that lies lateral to the neural crest. The neural crest domain and the PPR are established in response to signaling events mediated by BMPs, FGFs and Wnts, which differentially activate transcription factors in these territories. In the PPR, members of the Six and Eya families, act in part to repress neural crest specific transcription factors, thus solidifying a placode developmental program. Subsequently, in response to environmental cues the PPR is further subdivided into placodal territories with distinct characteristics, each expressing a specific repertoire of transcription factors that provide the necessary information for their progression to mature sensory organs. In this review we summarize recent advances in the characterization of the signaling molecules and transcriptional effectors that regulate PPR specification and its subdivision into placodal domains with distinct identities.
Keywords: BMP; Cranial sensory placodes; Eya; FGF; Gene regulatory network; Pax; Pre-placodal ectoderm; Six; Wnt.
Copyright © 2014 Elsevier Inc. All rights reserved.
Figures


References
-
- Abdelhak S, Kalatzis V, Heilig R, Compain S, Samson D, Vincent C, Weil D, Cruaud C, Sahly I, Leibovici M, Bitner-Glindzicz M, Francis M, Lacombe D, Vigneron J, Charachon R, Boven K, Bedbeder P, Van Regemorter N, Weissenbach J, Petit C. A human homologue of the Drosophila eyes absent gene underlies Branchio-Oto-Renal (BOR) syndrome and identifies a novel gene family. Nature Genet. 1997;15:157–164. - PubMed
-
- Abello G, Khatri S, Giraldez F, Alsina B. Early regionalization of the otic placode and its regulation by the Notch signaling pathway. Mech. Dev. 2007;124:631–645. - PubMed
-
- Ahrens K, Schlosser G. Tissues and signals involved in the induction of placodal Six1 expression in Xenopus laevis. Dev. Biol. 2005;288:40–59. - PubMed
-
- Altmann CR, Chow RL, Lang RA, Hemmati-Brivanlou A. Lens induction by Pax-6 in Xenopus laevis. Dev. Biol. 1997;185:119–123. - PubMed
-
- Arima K, Shiotsugu J, Niu R, Khandpur R, Martinez M, Shin Y, Koide T, Cho KW, Kitayama A, Ueno N, et al. Global analysis of RAR-responsive genes in the Xenopus neurula using cDNA microarrays. Dev. Dyn. 2005;232:414–431. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

