Effect of hypoxia on lung gene expression and proteomic profile: insights into the pulmonary surfactant response
- PMID: 24576641
- PMCID: PMC4017078
- DOI: 10.1016/j.jprot.2014.02.019
Effect of hypoxia on lung gene expression and proteomic profile: insights into the pulmonary surfactant response
Abstract
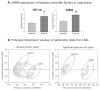
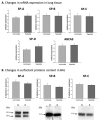
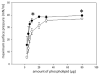
Exposure of lung to hypoxia has been previously reported to be associated with significant alterations in the protein content of bronchoalveolar lavage (BAL) and lung tissue. In the present work we have used a proteomic approach to describe the changes in protein complement induced by moderate long-term hypoxia (rats exposed to 10% O2 for 72h) in BAL and lung tissue, with a special focus on the proteins associated with pulmonary surfactant, which could indicate adaptation of this system to limited oxygen availability. The analysis of the general proteomic profile indicates a hypoxia-induced increase in proteins associated with inflammation both in lavage and lung tissue. Analysis at mRNA and protein levels revealed no significant changes induced by hypoxia on the content in surfactant proteins or their apparent oligomeric state. In contrast, we detected a hypoxia-induced significant increase in the expression and accumulation of hemoglobin in lung tissue, at both mRNA and protein levels, as well as an accumulation of hemoglobin both in BAL and associated with surface-active membranes of the pulmonary surfactant complex. Evaluation of pulmonary surfactant surface activity from hypoxic rats showed no alterations in its spreading ability, ruling out inhibition by increased levels of serum or inflammatory proteins.
Biological significance: This work reveals that hypoxia induces extensive changes in the proteomic profile of lung bronchoalveolar lavage, including the presence of proteins related with inflammation both in lung tissue and lavage, and a significant increase in the synthesis and secretion by the lung tissue of different forms of hemoglobin. The level of specific pulmonary surfactant-associated proteins is not substantially altered due to hypoxia, but hypoxia-adapted surfactant exhibits an enhanced ability to form surface-active films at the air-liquid interface. The increased amount of β-globin integrated into the operative surfactant complexes obtained from hypoxic rats is a relevant feature that points to the existence of adaptive responses coupling surfactant function and oxygen availability.
Keywords: ABCA3; Air–liquid interface; Hemoglobin; Lung surfactant; Surfactant proteins.
Copyright © 2014 Elsevier B.V. All rights reserved.
Figures




References
-
- Lopez-Barneo J, Pardal R, Ortega-Saenz P. Cellular mechanism of oxygen sensing. Annu Rev Physiol. 2001;63:259–87. - PubMed
-
- Jain M, Sznajder JI. Effects of hypoxia on the alveolar epithelium. Proc Am Thorac Soc. 2005;2:202–5. - PubMed
-
- Bhattacharya J, Matthay MA. Regulation and repair of the alveolar-capillary barrier in acute lung injury. Annu Rev Physiol. 2013;75:593–615. - PubMed
-
- Semenza GL. Pulmonary vascular responses to chronic hypoxia mediated by hypoxia-inducible factor 1. Proc Am Thorac Soc. 2005;2:68–70. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

