Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response
- PMID: 24576687
- PMCID: PMC4117827
- DOI: 10.1016/j.biopsych.2014.01.013
Enduring deficits in brain reward function after chronic social defeat in rats: susceptibility, resilience, and antidepressant response
Abstract
Background: Anhedonia, or diminished interest or pleasure in rewarding activities, characterizes depression and reflects deficits in brain reward circuitries. Social stress induces anhedonia and increases risk of depression, although the effect of social stress on brain reward function is incompletely understood.
Methods: This study assessed the following: 1) brain reward function in rats (using the intracranial self-stimulation procedure) and protein levels of brain-derived neurotrophic factor and related signaling molecules in response to chronic social defeat, 2) brain reward function during social defeat and long-term treatment with the antidepressants fluoxetine (5 mg/kg/day) and desipramine (10 mg/kg/day), and 3) forced swim test behavior after social defeat and fluoxetine treatment.
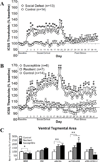
Results: Social defeat profoundly and persistently decreased brain reward function, reflecting an enduring anhedonic response, in susceptible rats, whereas resilient rats showed no long-term brain reward deficits. In the ventral tegmental area, social defeat, regardless of susceptibility or resilience, decreased brain-derived neurotrophic factor and increased phosphorylated AKT, whereas only susceptibility was associated with increased phosphorylated mammalian target of rapamycin. Fluoxetine and desipramine reversed lower, but not higher, stress-induced brain reward deficits in susceptible rats. Fluoxetine decreased immobility in the forced swim test, as did social defeat.
Conclusions: These results suggest that the differential persistent anhedonic response to psychosocial stress may be mediated by ventral tegmental area signaling molecules independent of brain-derived neurotrophic factor and indicate that greater stress-induced anhedonia is associated with resistance to antidepressant treatment. Consideration of these behavioral and neurobiological factors associated with resistance to stress and antidepressant action may promote the discovery of novel targets to treat stress-related mood disorders.
Keywords: Anhedonia; BDNF; ICSS; depression; mTOR; stress.
Copyright © 2014 Society of Biological Psychiatry. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Figures



Comment in
-
Chronic social defeat and intracranial self-stimulation: unmasking the many faces of depression?Biol Psychiatry. 2014 Oct 1;76(7):513-4. doi: 10.1016/j.biopsych.2014.04.008. Epub 2014 May 8. Biol Psychiatry. 2014. PMID: 24835177 No abstract available.
References
-
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. 5th ed. Arlington, VA: American Psychiatric Association; 2013.
-
- Loas G. Vulnerability to depression: a model centered on anhedonia. J Affect Disord. 1996;41:39–53. - PubMed
-
- Schrader GD. Does anhedonia correlate with depression severity in chronic depression? Compr Psychiatry. 1997;38:260–263. - PubMed
-
- Charney DS, Manji HK. Life stress, genes, and depression: multiple pathways lead to increased risk and new opportunities for intervention. Sci STKE. 2004;2004:re5. - PubMed
-
- Kendler KS, Karkowski LM, Prescott CA. Causal relationship between stressful life events and the onset of major depression. Am J Psychiatry. 1999;156:837–841. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

